
Rhythm Pharmaceuticals (NASDAQ:RYTM), with the only FDA-approved therapy that targets a root cause of severe obesity and insatiable hunger, is in clinical development to expand the addressable population of patients with rare genetic and acquired diseases of obesity.
“We are focused on the melanocortin-4 receptor (MC4R) pathway, which is responsible for regulating hunger, energy expenditure and weight,” David Meeker, M.D., chairman, president and CEO of Rhythm, says in an interview with BioTuesdays.
“Impairments of the MC4R pathway lead to excessive or insatiable hunger, or hyperphagia, and by targeting the MC4R pathway, we aim to develop a therapy for many rare diseases of obesity,” he adds.
Rhythm’s IMCIVREE, or setmelanotide, has been approved as the only therapy to treat certain rare genetic obesities as well as a syndromic obesity due to Bardet-Biedl syndrome. The continued clinical development of setmelanotide includes an ongoing Phase 3 trial in four specific genetically-caused obesities as well as a planned Phase 3 trial in 2023 in hypothalamic obesity, a rare, severe form of obesity that occurs as a consequence of certain brain cancers.
Rhythm’s first approval for IMCIVREE came from the U.S. FDA in 2020 when it was approved for obesity due to a deficiency in the POMC, PCSK1 or LEPR genes. The European Commission in 2021 also granted marketing authorization to IMCIVREE for the treatment of obesity and control of hunger associated with a genetically confirmed loss-of-function in the POMC, PCSK1 and LEPR genes.
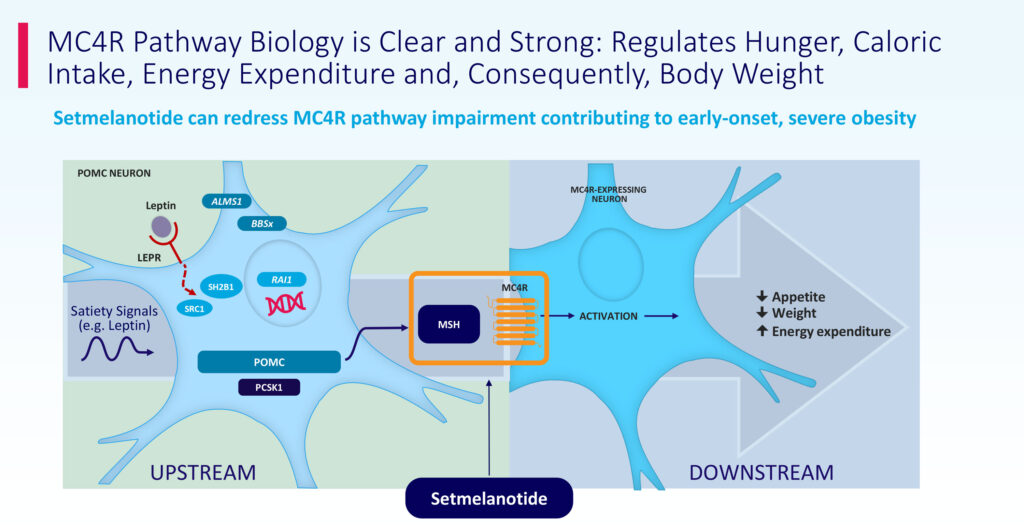
People living with obesity because of POMC, PCSK1 or LEPR deficiency struggle with extreme, insatiable hunger beginning at a young age, resulting in early-onset, severe obesity. As an MC4R agonist, IMCIVREE, is designed to restore impaired MC4R pathway activity arising because of genetic deficits upstream of the MC4R receptor, Dr. Meeker says.
These approvals were based on results from the largest studies ever conducted in obesity due to POMC, PCSK1 or LEPR deficiency. In Phase 3 clinical trials, 80% of patients with obesity due to POMC or PCSK1 deficiency achieved greater than 10% weight loss and 45.5% of patients with obesity due to LEPR deficiency achieved greater than 10% weight loss after one year of treatment with IMCIVREE.
Dr. Meeker says market access is being achieved in Europe country-by-country, including major markets in France and Germany, with progress in the UK, for the treatment of obesity and hyperphagia due to POMC, PCSK1 and LEPR deficiencies.
In June 2022, the FDA approved IMCIVREE for use in patients with Bardet-Biedl syndrome, a rare genetic disease that affects approximately 1,500-to-2,500 people in the U.S.
The EMA’s Committee for Medicinal Products for Human Use has adopted a positive opinion recommending to expand the current marketing authorization for IMCIVREE to include the treatment of obesity and control of hunger in adult and pediatric patients six years of age and older with genetically confirmed Bardet-Biedl syndrome. Final authorization for Bardet-Biedl syndrome is anticipated in the fourth quarter of 2022. The estimated European prevalence is about 2,500 patients.
“We think genetic testing in the future will identify many more undiagnosed patients,” Dr. Meeker suggests.
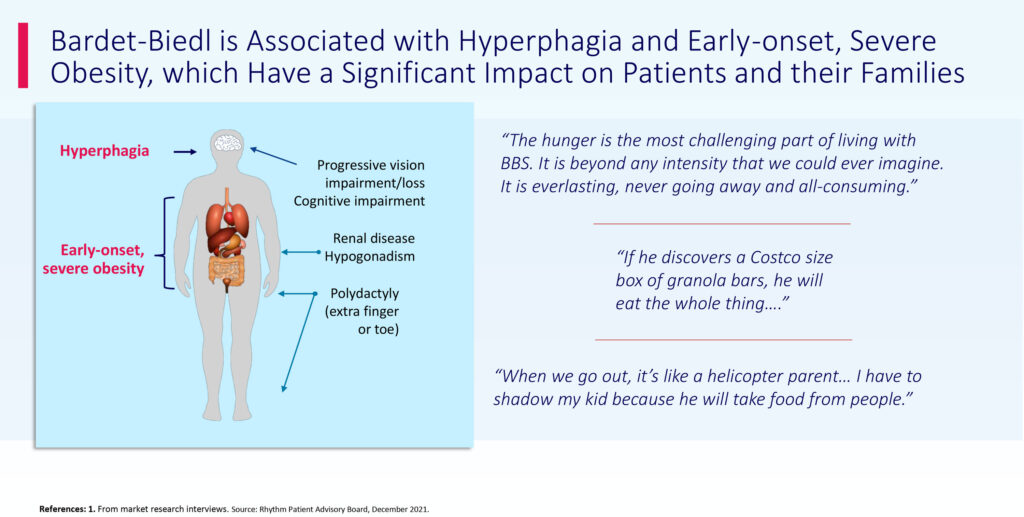
People living with Bardet-Biedl syndrome may experience insatiable hunger and severe obesity beginning early in life. Bardet-Biedl syndrome also may be associated with cognitive impairment, renal dysfunction, hypogonadism, visual impairment and polydactyly, which refers to extra fingers or toes at birth. In Rhythm’s 52-week pivotal study for BBS, 11-of-28 patients with Bardet-Biedl syndrome achieved 10% reduction in body weight.
In July 2022, Rhythm reported positive interim results from a Phase 2 clinical trial evaluating setmelanotide, its MC4R agonist, for the treatment of severe obesity and hyperphagia in people living with hypothalamic obesity. Based on the data, the company intends to proceed to Phase 3 clinical development following consultation with regulatory agencies.
Dr. Meeker suggests the initial results reinforce the importance of the MC4R pathway in regulating hunger, caloric intake, energy expenditure and ultimately body weight, as well as the potential role of setmelanotide in the management of diseases where this pathway is impaired.
Hypothalamic obesity is a rare, acquired form of extreme obesity that occurs following damage to the hypothalamic region of the brain, which is responsible for controlling physiological functions, such as hunger and weight regulation. Rhythm estimates there are 5,000-to-10,000 patients living with hypothalamic obesity in the U.S., with approximately 500 new cases each year.
Rhythm is now in clinical development to expand IMCIVREE’s label and patient population with additional genes linked to the MC4R pathway. The company is currently conducting the Phase 3 EMANATE trial, which includes four independent sub-studies evaluating setmelanotide in patients with obesity due to a heterozygous variant of the POMC/PCSK1 genes, the LEPR gene, the SRC1 gene and the SH2B1 gene.
According to Dr. Meeker, Rhythm estimates that patients with rare variants in these genes represent a potential addressable U.S. population of approximately 53,000, based on internal genetic sequencing data.
Rhythm enrolled the first patient in the EMANATE trial in April 2022 and anticipates 12-to-18 months to enroll approximately 400 patients in the trial. EMANATE will enroll patients with hyperphagia and obesity that began in early childhood. In each of the four sub-studies, patients will be randomized one-to-one to daily treatment with setmelanotide or placebo.
The primary efficacy endpoint in each sub-study is the mean change from baseline to 52 weeks in body weight, assessed as percent change in body mass index in response to setmelanotide, compared with placebo.
Rhythm also is conducting the Phase 2 DAYBREAK trial to focus on rare variants associated with 10 MC4R-relevant genes that it believes have the highest probability of success, Dr. Meeker says.
Rhythm began enrolling DAYBREAK in January 2022. DAYBREAK is a two-stage trial, beginning with a 16-week open-label stage followed, for patients who demonstrate a clinically meaningful response to setmelanotide, by a 24-week double-blind, placebo-controlled stage.
The trial will enroll approximately 100-to-200 patients with hyperphagia and severe obesity and a variant in one of 10 genes. Dr. Meeker says the company believes this two-stage design is an efficient way to assess clinically meaningful response to setmelanotide. Each genetically defined cohort can read out results independently.
Setmelanotide also is being evaluated in pediatric patients, ages two-to-six years old, with obesity due to Bardet-Biedl syndrome or genetically confirmed loss-of-function in the POMC, PCSK1 and LEPR genes. The weekly formulation would extend setmelanotide’s IP and improve patient compliance.
Rhythm had $241-million of cash at March 31, 2022, which is expected to fund operations at least into the second half of 2024, Dr. Meeker points out.
He says the company is in the early stages of its commercial launch in the U.S. with IMCIVREE for the treatment of Bardet-Biedl syndrome. “The BBS community is ready, physicians are engaged and patients have been identified. And payers are receptive to IMCIVREE for BBS with strong coverage expected,” he adds.
The commercial plan includes territory sales managers who are each focused on connecting with more than 350 identified and diagnosed Bardet-Biedl syndrome patients and the 150 physicians who treat them in the U.S.
The company also has established a patient services group, known as Rhythm InTune to provide personalized support and education ahead of the start of therapy. “Call is support made personal,” he says.
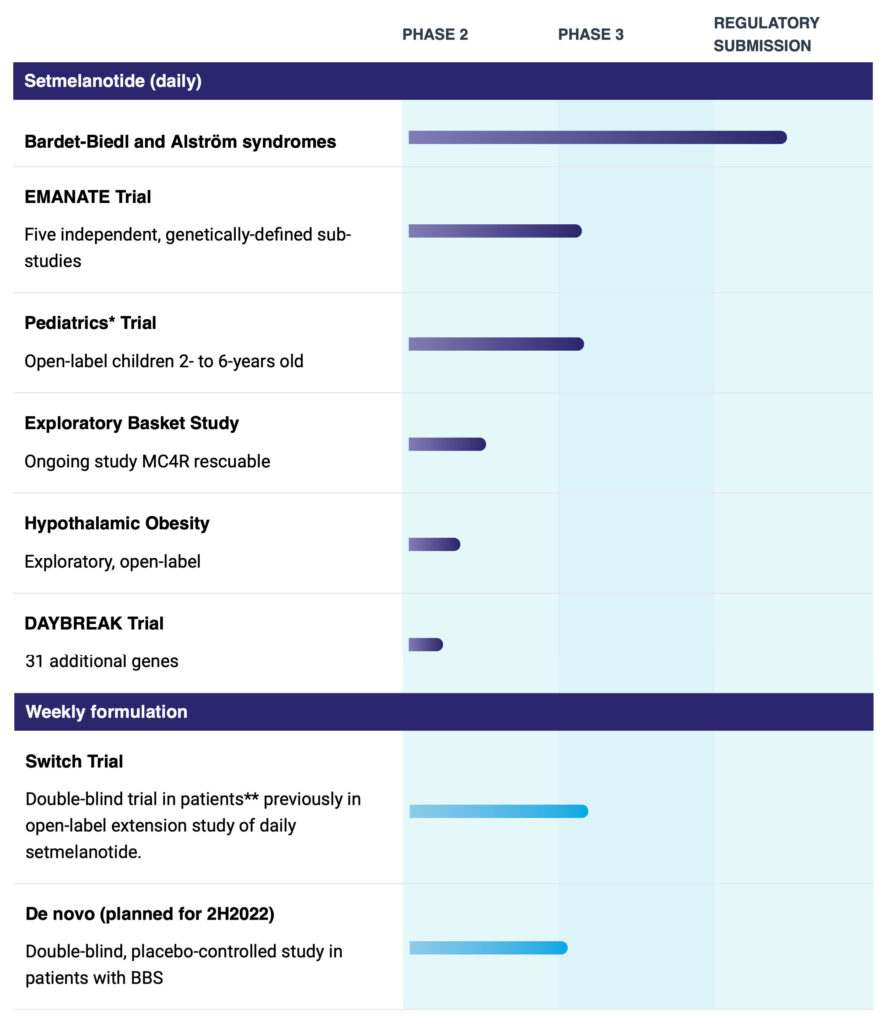
*Enrolled in the pediatrics trial are patients with obesity due to biallelic POMC, PCSK1 or LEPR deficiency or a clinical diagnosis of BBS with genetic confirmation.
**Enrolled in the switch trial are patients with obesity due to biallelic or heterozygous POMC, PCSK1 or LEPR deficiency or a clinical diagnosis of BBS with genetic confirmation.
• • • • •
To connect with Rhythm Pharmaceuticals or any of the other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.







