TC BioPharm (NASDAQ:TCBP) is a global leader in developing platform allogeneic gamma-delta T-cell therapies for cancer indications, with human efficacy data in acute myeloid leukemia (AML) and plans to expand into other blood cancers.
“Gamma-delta T-cells constantly monitor the body for signs of biological stress and are our first defense against disease with an inherent ability to locate and then kill stressed cells,” Bryan Kobel, CEO of TC BioPharm, says in an interview with BioTuesdays.
Mr. Kobel explains that all diseased cells, including cancer tumors, emit a very specific phosphoantigen called, IPP, or isopentenyl pyrophosphate. Healthy cells do not emit IPP and are not targeted by gamma-delta T-cells, making them non-toxic and safe for non-cancer cells. All tumors discovered emit IPP, making the mechanism of action for gamma deltas interesting, given its potential universal nature to all cancers.
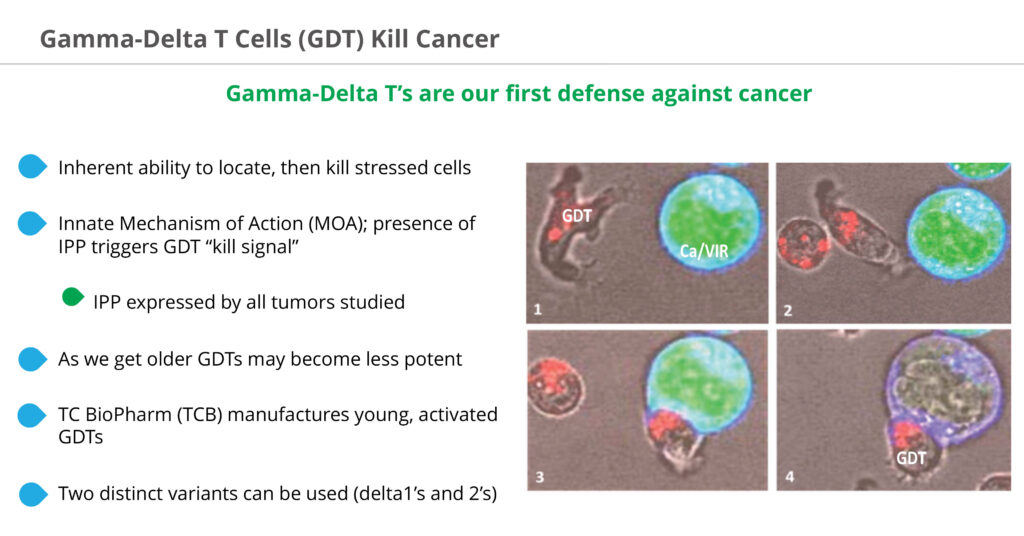
“IPP is what draws the gamma-deltas to a cell to affect apoptosis, or cell death, much the way sharks smell blood in the water,” he adds. “IPP is the trigger for the gamma-deltas to say this is bad for the body. We know gamma-deltas are able to kill tumor cells, but the issue is whether we can get enough of the activated gamma-deltas to the tumor.”
Mr. Kobel points out that as people age, become sick or immuno-compromised, their gamma-delta T-cells become less potent and there aren’t enough of them in the body to fight disease.
TC BioPharm manufactures young, active gamma-delta T-cells exogenously using donor blood, expanding the gamma-delta T-cell population into the billions and infusing these healthy donor cells into cancer patients. Gamma deltas do not create a graft versus host disease issue, making them ideal for allogeneic delivery into sick patients.
The company was founded in 2014 by Dr. Mike Leek, a pioneer in regenerative medicine, and Angela Scott, who played a key role in creating the first animal clone, Dolly the Sheep, in 1996. The company is headquartered in Edinburgh, Scotland, has more than 70 employees and is in clinical stage Phase 2b.
Mr. Kobel says TC BioPharm is vertically integrated, with product development, manufacturing and quality functions outside Glasgow. Facilities in London, Edinburgh and the U.S. support its global clinical efforts and strategic growth.
“The biggest issue in cell therapy today is the supply-demand bottleneck. For a cell therapy company without manufacturing, it can take up to a year to order a batch of cells to run a clinical study. This is costly and there can be quality issues with the cells,” he adds.
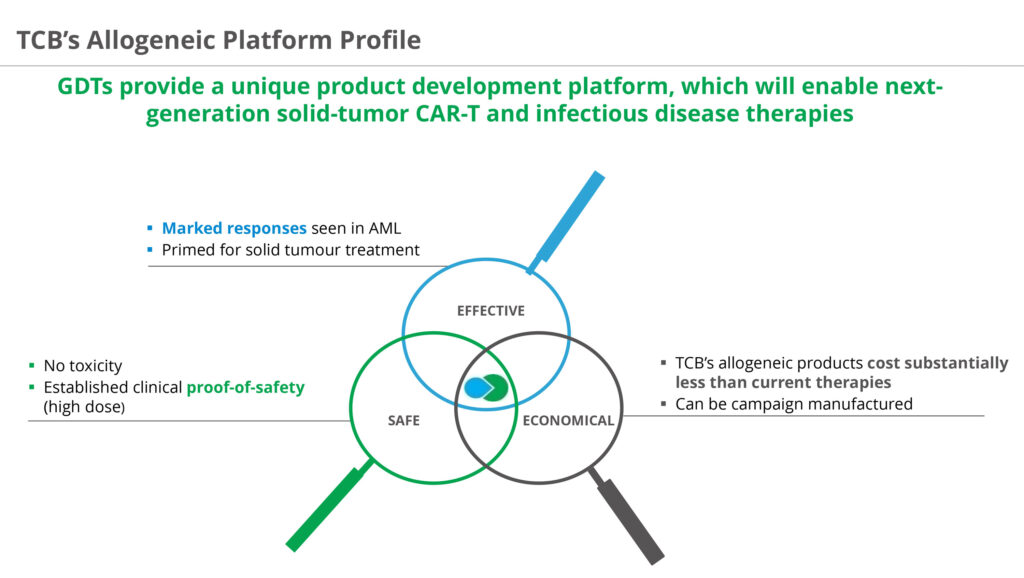
“By vertically integrating, we can move faster from the lab bench to human data than companies that outsource their cell product. And by working with these living organisms every day, our knowledge base expands about how to make these cells more effective in oncology indications at a significant costs savings than outsourcing.”
Mr. Kobel says the company plans to build a manufacturing plant in the U.S. for its cell therapy platform as well as seeking additional partnerships. In 2017, TC BioPharm inked a collaboration with bluebird bio, which has been renamed, 2seventy bio, to advance its chimeric antigen receptor (CAR) gamma-delta T-cell program into clinical oncology trials.
“We hope to conduct site visits and make some decisions about manufacturing in the U.S. by the end of 2022.”
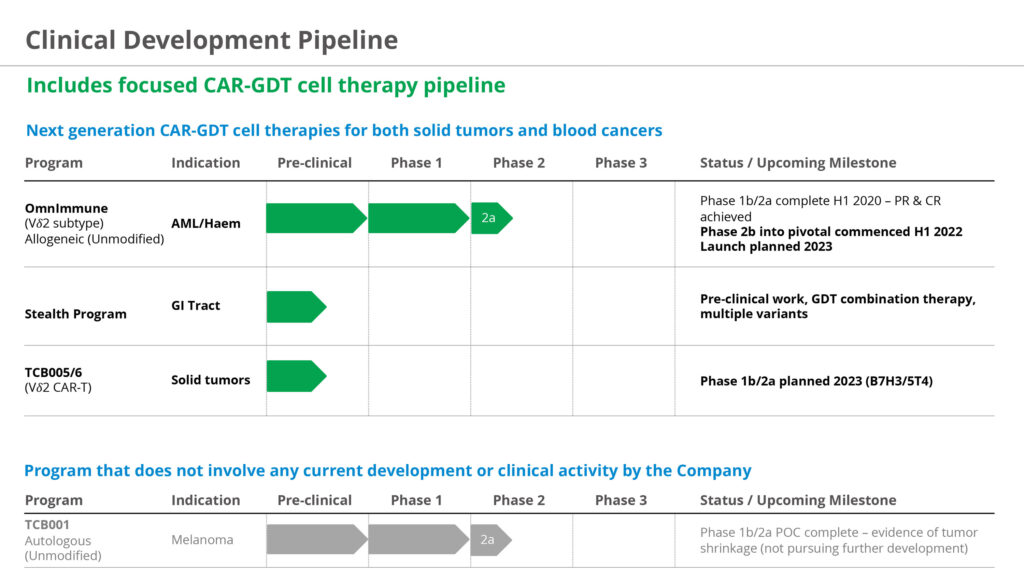
In March 2022, the company publicly reported positive interim data from its Phase 1b/2a human study in Prague evaluating the safety and tolerability of OmnImmune, its lead allogenic gamma-delta T-cell product, for the treatment of relapsed/refractory AML and other blood cancers. Patients in the study had poor life expectancy and prior clinical options had failed.
Of the seven patients treated in the study, three received a single dose of OmnImmune at a low dose of cells and four received a single dose of OmnImmune at a medium dose of cells.
In a medium-dose cohort, 50% of patients achieved a complete response with one patient achieving a morphologic leukemia-free state and one patient received a complete response; of the remaining two, one patient showed disease progression and one patient exhibited significantly reduced cancer blast count at day 14 before the trial was cut short due to Covid.
In the low dose cohort, one patient achieved stable disease, one patient had progressive disease and one patient met safety endpoints but was lost to follow-up due to pneumonia unrelated to OmnImmune treatment.
According to Mr. Kobel, the Phase 1b/2a clinical trial showed that average cancer levels in bone decreased from 38% to 6%, with no serious adverse treatment-related safety events. OmnImmune received orphan drug status from the FDA in March 2022. Additionally, several of the patients received repeat dosing, showing the tolerance of the therapeutic and seeing no toxicity associated.
Based on compelling clinical data in non-responding patients, the company has progressed to a Phase 2b/3 clinical trial at sites in the UK and Europe for patients that have failed first-line chemotherapy. The ultimate goal of a study is a bridge to a bone marrow transplant.
TC BioPharm has advanced the product to frozen/thawed, meaning they can now ship and store OmnImmune at clinical sites to provide a true “off the shelf” cell therapy. In addition, the Phase 2b/3 trial will not “HLA match,” moving away entirely from donor matching. The first cohort of patients in the UK is 19 patients with the first five being considered further safety measuring.
“However, if we see the response we’re hoping for, there might not be a need for a bone marrow transplant and these patients could be treated with additional doses of gamma-deltas,” Mr. Kobel suggests.
The company plans to expand the Phase 2/3 study to the U.S. in the fourth quarter of 2022 or early 2023. Currently, the company is in conversations with the FDA regarding the final trial protocol in conjunction with its U.S. clinical partner.
TC BioPharm also is considering a combination trial in the U.S. with gamma-deltas in combination with venetoclax, a chemotherapy, to treat AML in 20-to-30 patients. “This would most likely be a relapsed/refractory trial, moving to first-line treatment that we hope to launch in the first half of 2023,” he adds.
“2023 will be an exciting year for TC BioPharm,” Mr. Kobel contends. “On the clinical side, we hope to complete our AML trial in the UK and EU; begin enrolling our U.S. AML trial cohort, and meet with the FDA about an IND to advance our gamma-delta CAR-T program in solid tumors. And we also plan to expand our gamma-delta platform into other indications, such as acute lymphocytic leukemia, chronic lymphocytic leukemia and multiple myeloma.”
• • • • •
To connect with TC BioPharm or any of the other companies featured on BioTuesdays, send us an email at [email protected].