
MindMed (NASDAQ:MNMD; NEO:MMED) is advancing a pipeline of psychedelic and next generation drug candidates to treat brain health disorders, with a particular focus on psychiatry, addiction, pain and neurology.
“Our mission is to be the global leader in the development and delivery of potential psychedelic treatments that unlock new opportunities to improve patient outcomes,” Robert Barrow, CEO of MindMed, as the company is known, says in an interview with BioTuesdays.
“We are developing a pipeline of innovative drug candidates, with and without acute perceptual effects, based on psilocybin, LSD, MDMA, DMT and an ibogaine derivative, targeting the serotonin, dopamine and acetylcholine systems,” he adds. “Moreover, our product candidates have an abundance of scientific evidence, both current and historical, on their potential as treatments for brain health disorders.”
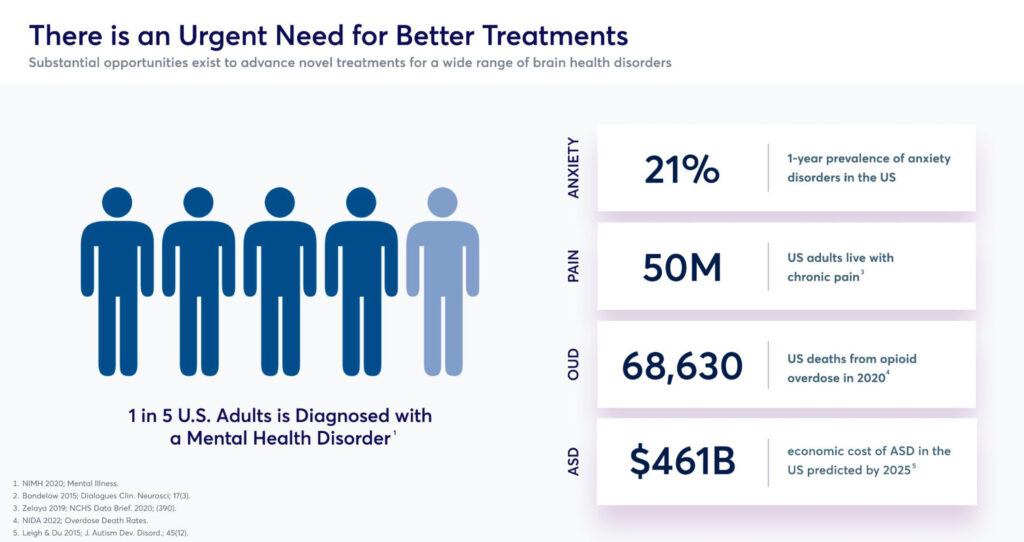
Mr. Barrow says there is an urgent need for innovation in this field and better treatments. According to the National Institute of Mental Health, one-in-five U.S. adults is diagnosed with a mental health disorder, which covers anxiety, pain, opioid use disorder and autism spectrum disorder, which is predicted to reach an economic cost of $461-billion in the U.S. by 2025.
MindMed has three active product candidates in its pipeline including:
- MM-120, an LSD tartrate, MindMed’s lead program, in development for generalized anxiety disorder, attention deficit hyperactivity disorder (ADHD) and chronic pain.
- MM-110, a nicotinic cholinergic receptor antagonist that offers a novel mechanism to potentially address a critical gap in current opioid use disorder treatment.
- And MM-402, an enantiomer of MDMA that exhibits prosocial and emotional openness activity in development for the treatment of core symptoms of autism spectrum disorder (AUD).
“The question we get asked regularly is how can you have intellectual property protection for a molecule like LSD that has been known for 75 years and has been clinically administered to more than 10,000 patients? Of course, we don’t have composition of matter patents on LSD, but we have developed advanced formulations and other innovations for our active pharmaceutical ingredient, establishing layers of IP protection that would give us the ability to protect any future market share,” Mr. Barrow points out.
As a potent serotonin agonist, MM-120 has demonstrated overall and entropic brain connectivity and increased single neuron excitability, he adds. “There is extensive evidence of the clinical benefit and mechanistic rationale for the compound in psychiatry, pain and substance use disorders.”
At London’s PSYCH conference earlier this month, MindMed collaborators at University Hospital Basel presented positive topline data from a Phase 2 placebo-controlled investigator-initiated clinical trial evaluating LSD in 42 patients for the treatment of anxiety disorders.
“These results represent the highest quality research ever conducted with LSD in anxiety disorders,” Mr. Barrow contends, adding that they also confirm preliminary findings of the anxiety reduction and antidepressant effects of LSD. MindMed recently initiated a Phase 2b placebo-controlled study with MM-120 for the treatment of generalized anxiety disorder.
MindMed is attempting to demonstrate dose-responsive effects in the Phase 2b study and identify an optimal dose of MM-120 for pivotal trials, as well as durability of effect, out to 12 weeks. The study will enrol 200 men and women aged 18-to-74 in four treatment arms and placebo at 20 sites in the U.S. Patients in the treatment arms will receive either 25, 50, 100 or 200 micrograms of MM-120.
In December 2021, MindMed began evaluating MM-120 in a Phase 2a proof-of-concept study for the treatment of ADHD. The study, at two sites in Switzerland and the Netherlands, is designed to assess the safety and efficacy of administering repeated low-doses of MM-120.
Twenty-six patients in the treatment arm will receive 20 micrograms of MM-120 twice weekly and 26 patients will receive a placebo. The study will evaluate primary and secondary endpoints using the ADHD investigator symptom rating scale.
The anxiety and ADHD studies are expected to readout in the second half of 2023.
Mr. Barrow says there is legacy preclinical and early clinical evidence to support the use of LSD in terminal cancer pain and phantom limb pain as well as in pain tolerance in healthy volunteers. In the fourth quarter of 2022, MindMed expects to begin a Phase 2 study with MM-120 in chronic pain, measuring changes in daily pain on an 11-point numerical rating scale.
In its opioid withdrawal program, the company’s MM-110 molecule, or zolunicant, is an alpha-3-beta-4 nicotinic receptor antagonist with a differentiated mechanism of action that modulates excessive dopamine fluctuations in the mesolimbic system of the brain.
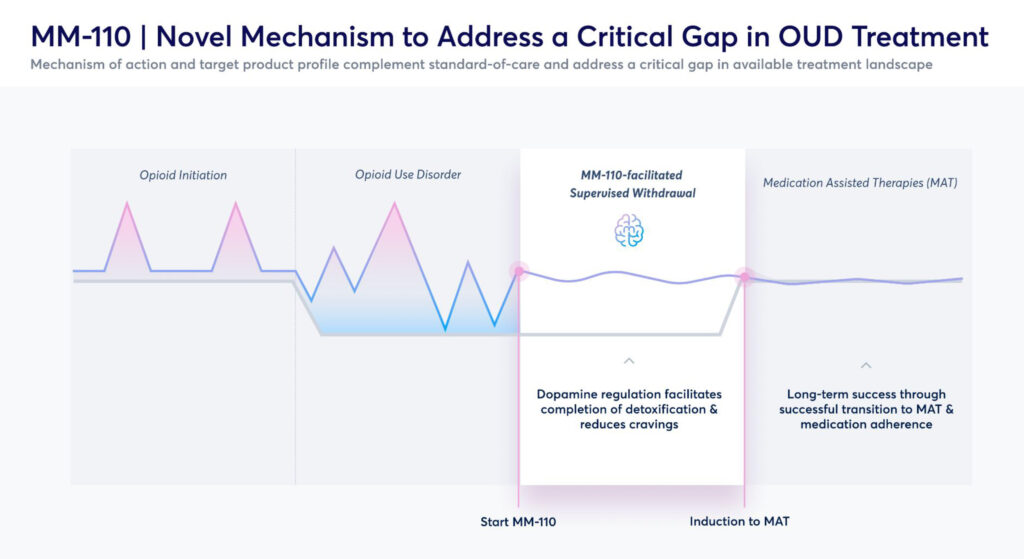
In preclinical efficacy models, Mr. Barrow says MM-110 demonstrated strong activity in reducing both withdrawal symptoms and self-administration of opioids, stimulants and other substances of abuse, as well as a strong safety and tolerability profile.
Earlier this month, MindMed reported favorable safety and tolerability results from a Phase 1 placebo-controlled trial designed to assess safety, tolerability, pharmacokinetics and neurocognitive effects of MM-110 in 108 healthy volunteers. A Phase 2a trial is set to begin in the current quarter.
The two-stage Phase 2a study will include an open label arm with 10 patients to determine early signs of efficacy in opioid withdrawal, followed by an interim analysis in the first half of 2023. The study would then move into part B, which would be a randomized, placebo-controlled proof-of-concept study of opioid withdrawal with 42 patients.
In its autism spectrum disorder program, Mr. Barrow says there are no approved drugs for the “core symptoms” of ASD that align with patient preferences. He says an FDA workshop on the subject found individuals living with ASD expressed their greatest needs as improving social functioning and communications difficulties, along with improved relationships to enhance the quality of life.
Based on historical data, he notes that pilot clinical trial results of MDMA have demonstrated acute and durable positive effects on social functioning in an ASD population. “We believe the pharmacological profile of MM-402 aligns with patient-desired treatment benefits.”
IND-enabling studies are currently ongoing MM-110 and, through MindMed’s collaboration with University Hospital Basel, a comparative Phase 1 pharmacokinetic/pharmacodynamic study of several formulations of MDMA with healthy volunteers is expected to begin in the third quarter of 2022.
In early 2021, MindMed launched a digital medicine division following the acquisition of HealthMode, a digital medicine and therapeutics company that uses artificial intelligence-enabled digital measurement to increase the precision and speed of clinical research and patient monitoring.
“We are building an integrated technical platform with the aim of delivering psychedelic-derived medicines and experimental therapies combined with digital therapeutics,” Mr. Barrow says.
“Pairing digital tools and the latest in machine learning, with psychedelic assisted therapies, can give healthcare providers the ability to optimize and better understand the patient journey and therapeutic outcomes from pre-care through to after-care.”
The company has several studies underway with its Session Monitoring System to evaluate the passive collection of sensory data and quantifying the processes and events of psychotherapy at scale. A newly developed mobile application is evaluating anxiety digital diagnoses for precision psychiatry.
Mr. Barrow says the digital medicine division will not only build apps, technologies and other platforms to help the patient, but hopefully also make the medical community comfortable with this novel treatment paradigm for mental health and addiction by measuring the potential value to their patient populations and ultimate savings to insurers.
• • • • •
To connect with MindMed or any of the other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.







