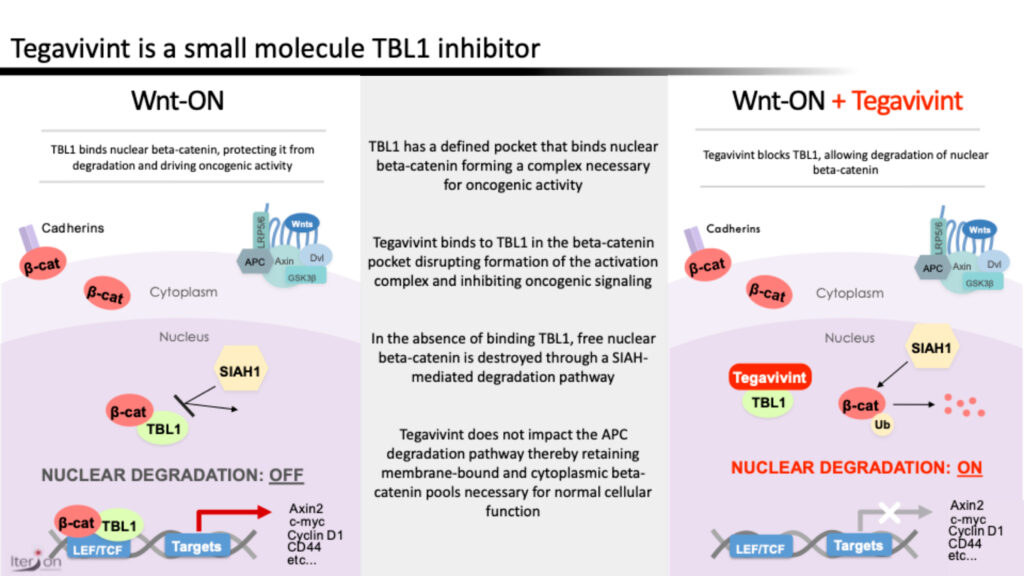
Closely-held Iterion Therapeutics is employing a unique strategy with its lead asset, tegavivint, which is designed to bind to TBL1 (transducin beta-like protein one) and inhibit nuclear beta-catenin signaling and oncogenic activity.
“We are one of the only biotechs pursuing TBL1 inhibition as a route to addressing multiple cancers,” Rahul Aras, Ph.D., president and CEO of Iterion, says in an interview with BioTuesdays.
Increased expression of beta-catenin and TBL1 are associated with metastasis and poor prognosis in a broad range of tumor types.
Dr. Aras explains that TBL1 has a defined pocket that binds nuclear beta-catenin, forming a complex necessary for oncogenic activity. “Tegavivint is designed to bind TBL1, inhibiting complex formation. This results in degradation of nuclear beta-catenin, without disrupting key cell membrane functions that have been linked to toxicity common to other drug candidates in this pathway.”
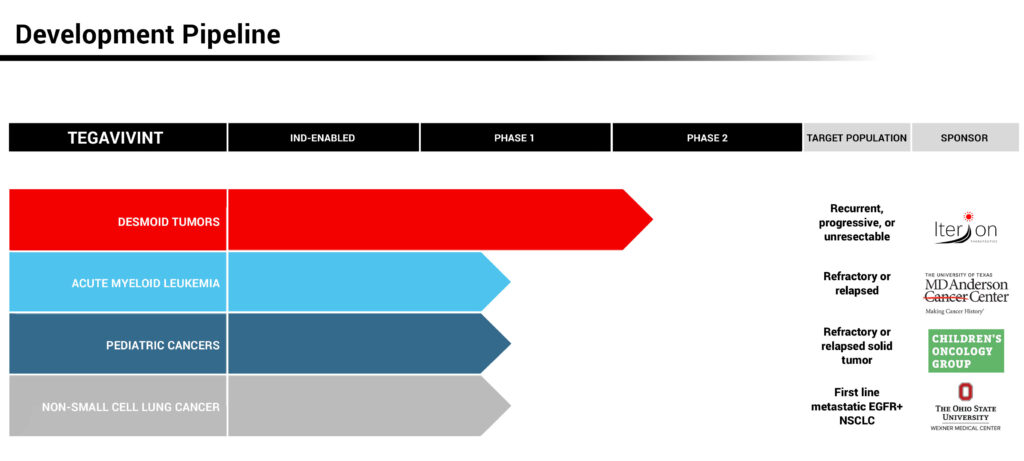
In a first-in-man Phase 1 clinical study with tegavivint in 24 patients with actively growing desmoid tumors, no dose limiting toxicities were observed, with several patients on treatment for longer than a year, without drug-related complications.
In addition, four patients who had failed prior treatment reported a more than 50% reduction in tumor volumes, with several patients achieving stable disease for a year or longer.
The study, which was conducted at MD Anderson Center, Fred Hutchinson Cancer Research Center, Memorial Sloan Kettering, Dana-Farber, Ohio State University and Princess Margaret Cancer Centre in Toronto, also established a recommended dose for Phase 2 studies.
Desmoid tumors are non-metastatic, painful fibrotic growths. There is no approved treatment for desmoid tumors, which do not have a high mortality but impact quality of life.
“We pursued desmoid tumors because 80% to 90% of patients have a mutation in beta-catenin, and patients are generally young, healthy individuals, so it was an opportunity to measure toxicity and clinical activity in a meaningful proof-of- concept study,” Dr. Aras recalls.
At the American Association for Cancer Research annual meeting in April, Iterion presented three posters about the Phase 1 study and tegavivint’s therapeutic potential.
“Taken together, these results demonstrate inhibition of TBL1 as a clinically viable strategy to curtail nuclear beta-catenin oncogenic activity in multiple cancer indications,” Dr. Aras contends.
In the fourth quarter of 2021, three investigator-sponsored studies began enrolling patients with tegavivant. They include:
- A Phase 1/2a clinical trial in patients with relapsed or refractory acute myeloid leukemia (AML) sponsored by MD Anderson Cancer Center.
- A Phase 1 clinical trial sponsored by Children’s Oncology Group Pediatric Early Phase Clinical Trials Network in pediatric patients with sarcomas, lymphomas and other solid tumors, excluding central nervous system tumors.
- And a Phase 1 clinical trial sponsored by Ohio State University Comprehensive Cancer Center in epidermal growth factor receptor-positive, non-small cell lung cancer (EGFR-NSCLC).
Dr. Aras says beta-catenin is a biomarker for poor prognosis in AML because it plays an important role in survival and growth of AML stem and progenitor cells that can lead to relapse. “In AML preclinical models, tegavivant has shown single agent and combination efficacy with approved chemotherapy, without inhibiting normal stem cells,” he adds.
The Phase 1 study at MD Anderson will determine single agent safety and activity, with the Phase 2a arm studying a combination of tegavivant and decitabine, a chemotherapy drug, in 35 relapsed/refractory AML patients. Results are expected in late 2022 or early 2023.
Dr. Aras says beta-catenin and TBL1 also are associated with metastasis and poor prognosis in a broad range of tumor types that are predominately found in children. And tegavivint has demonstrated single-agent and combination anti-tumor efficacy in multiple preclinical sarcoma and lymphoma models.
The Children’s Oncology Group at the NCI is conducting a Phase 1 study with tegavivint at their 21 pediatric cancer centers in the U.S. Data from the part A dose escalation study are expected later in 2022, with part B of the study measuring efficacy data in a broad range of pediatric cancers. “However, the main value of the study is pediatric safety data,” he adds.
Iterion’s third oncology program is measuring tegavivint’s ability to prevent enrichment of “drug-tolerant persister cells” in a Phase 1 study with 12-to-18 metastatic EGFR-NSCLCpatients at The Ohio State University Comprehensive Cancer Center.
Dr. Aras explains that some first-line tyrosine kinase inhibitors, such as osimertinib, enrich certain persister cells, leading to eventual development of drug resistance. Researchers at Ohio State have demonstrated that enrichment of persister cells by osimertinib is driven by activation of beta-catenin, and that tegavivint in combination with osimertinib prevented enrichment of these persister cells.
“This trial has the potential to help an enormous patient population and further demonstrate tegavivint’s unique mechanism of action of TBL1 inhibition, thereby disrupting the oncogenic activity of beta-catenin,” Dr. Aras contends.
• • • • •
To connect with Iterion Therapeutics or any of the other companies featured on BioTuesdays, send us an email at [email protected].