
After a failed Phase 3 trial in August 2020 with its CaPre lead drug candidate for patients with severe hypertriglyceridemia, Acasti Pharma (NASDAQ, TSXV:ACST) has transitioned into the rare and orphan disease sector with three lead clinical compounds, targeting significant unmet medical needs.
“Despite not meeting the primary endpoints, the triglyceride reduction with CaPre, which was developed using omega-3 fatty acids derived from krill oil, was one of the highest seen among previously conducted hypertriglyceridemia studies, particularly in patients on statins,” Jan D’Alvise, president and CEO, says in an interview with BioTuesdays.
“However, we had a very large placebo effect, which we’ve since figured out, but didn’t have the cash to repeat a pivotal trial last year.”
Ms. D’Alvise says the company has received strong interest in CaPre and continues to evaluate a variety of strategic options for its shelved asset.
The board engaged Oppenheimer in September 2020 to explore strategic opportunities for Acasti, which evaluated more than 100 companies, and selected Grace Therapeutics based on its novel drug delivery technologies and deep pipeline of clinical and non-clinical assets.
The all-stock acquisition of Grace was completed in August 2021, and the transaction gave the new company a foothold in three underserved orphan diseases: subarachnoid hemorrhage (SAH), ataxia-telangiectasia (AT) and postherpetic neuralgia (PHN) – all with orphan disease designations, sizeable patient populations and significant market opportunities, Ms. D’Alvise points out. Acasti also gained a portfolio of preclinical assets in the rare disease space.
In addition, Acasti had raised approximately $60-million through its at-the-market offering when biotech markets soared at the beginning of 2021, and that capital is now being used to advance the clinical pipeline.
“We ended up in a very different place than we envisioned a year ago,” she contends.
According to Ms. D’Alvise, who moved into the executive suite in mid-2016, orphan diseases generally command premium pricing, utilize formulations and compounds with proven safety, and by pursuing a 505(b)(2) regulatory pathway, “we may streamline how many clinical trials we need to conduct.”
The company has an IP portfolio with more than 40 granted and pending patents worldwide that provide exclusivity beyond 2036.
Subarachnoid hemorrhage (SAH) is a rare and life-threatening medical emergency in which bleeding, most commonly from an aneurysm, occurs over the surface of the brain in the subarachnoid space between the brain and skull. Some 10% to 15% of patients die before reaching hospital, while death or dependence affects 70% of patients.
The condition affects some 50,000 individuals in the U.S. and a similar number in Europe, with an estimated addressable market of more than $300-million in the U.S. alone.
Ms. D’Alvise says that at about $220,000 average cost per patient per hospital stay, SAH is one of the most expensive acute conditions to treat. Nimodipine, which was cleared by the FDA in 1988, is the only drug available to treat SAH but is known to cause very large drops in blood pressure, and must be carefully delivered and monitored, she adds.
In addition, nimodipine is only available as an oral capsule or liquid solution in the U.S, adding to the difficulty of blood pressure management, most notably when a patient is unconscious.
GTX-104: Superior Value Proposition
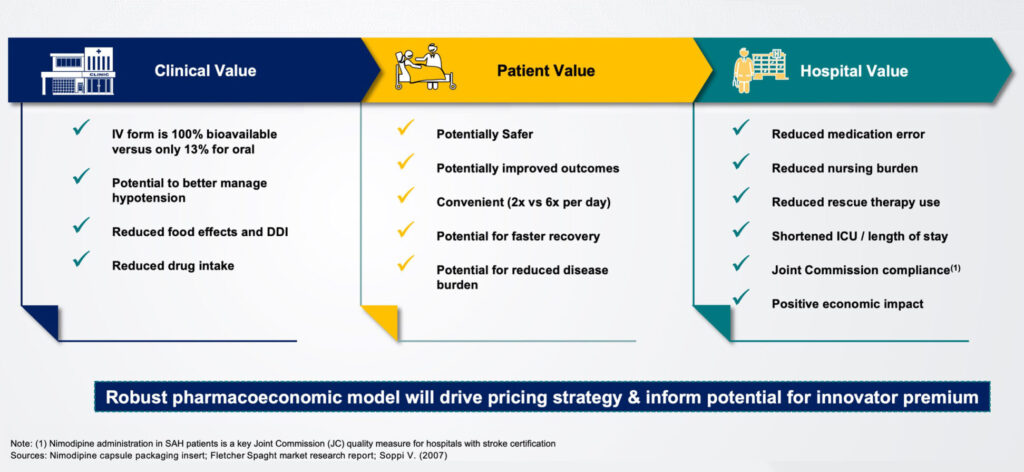
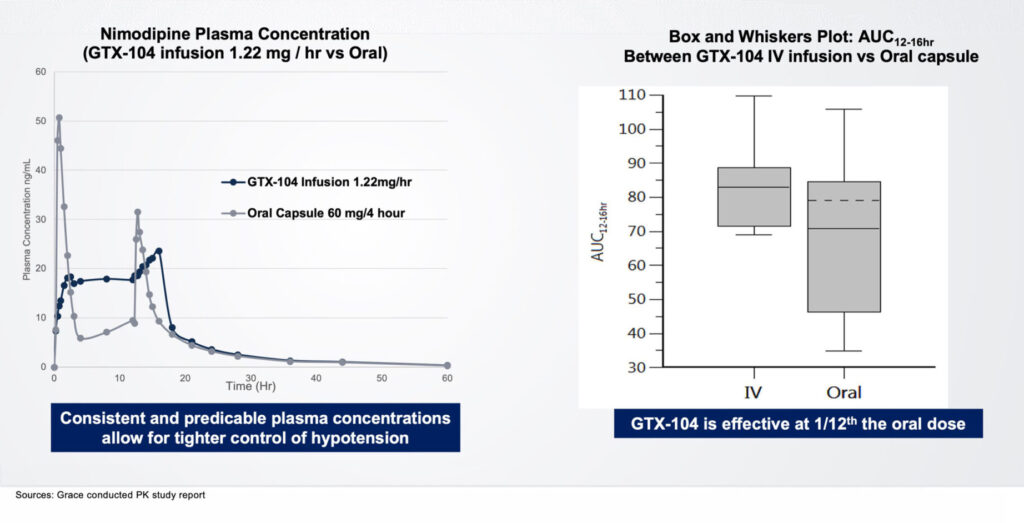
Acasti’s lead asset, GTX-104, is an IV-formulation of nimodipine that the company believes can potentially improve the management of hypotension and vasospasm, and outcomes, in SAH patients, and potentially prevent death and reduce long-term disability. “The IV form is 100% bioavailable, compared with only 13% for oral nimodipine,” Ms. D’Alvise contends.
In addition, she points out that GTX-104 is more convenient with twice-daily administration, compared with four-to-eight a day with nimodipine. She also notes that GX-104 has the potential for a positive economic impact by reducing medication errors; nursing burden and use of rescue therapy; and shortening the length of stay in the ICU.
GTX-104: Novel Nanoparticle Formulation for IV Infusion
Acasti expects to release results of a 50-patient pharmacokinetic study with GTX-104 in the first half of 2022 and, if the results are compelling, plans to start a Phase 3 safety study in the second half of 2022. “We could be on the market with GTX-104 by 2025.”
Ms. D’Alvise figures the company’s current cash position of approximately $60-million will fund GTX-104 through potential approval, as well as its next-in-line assets, GTX-102 and GTX-101, and other drug candidates to key milestones.
GTX-102 is Acasti’s novel oral spray formulation of betamethasone intended to improve neurological symptoms of ataxia-telangiectasia (A-T), a complex genetic neurodegenerative disorder often diagnosed during infancy or early childhood.
A-T is typically characterized by progressively impaired coordination, reddish lesions of the skin, impaired functioning of the immune system causing increased susceptibility to infection, and a predisposition to various cancers.
Ms. D’Alvise says A-T is inherited as an autosomal recessive trait and often affects more than one child in a family, with patients having a life span of about 25 years. A-T affects an estimated 4,300 individuals in the U.S. and has a potential total addressable U.S. market of $150-million, she adds.
There are no drugs approved for A-T and treatment is primarily directed at controlling symptoms through speech, occupational and physical therapy.
Ms. D’Alvise says Acasti has an exclusive, perpetual license to data from a study in 13 children with A-T by Dr. Raffaella Zannolli in Italy, which demonstrated that drinking oral betamethasone improved posture and gait, kinetic function and speech, but did not improve oculomotor disorders.
“On the basis of Dr. Zannolli’s study, we reformulated oral betamethasone into a concentrated oral spray and found in a preliminary pharmacokinetic study, that the volume of the mucosal spray that achieved target blood levels was 1/70th the volume of the oral solution used in the Zannolli study.”
Acasti is now working with investigators at John Hopkins University School of Medicine, which treats about one-half of all A-T patients in the U.S. Results from a new pharmacokinetic bridging study are expected in the second half of 2022, with a projected start of a Phase 3 study in the first half of 2023. “Our oral spray has the potential to be the first approved treatment for this indication,” Ms. D’Alvise contends.
Acasti’s third orphan drug candidate, GTX-101, is a bioadhesive film forming topical spray formulation of bupivacaine for the treatment of postherpetic neuralgia (PHN), a persistent and often debilitating neuropathic pain caused by nerve damage from the varicella zoster virus (shingles), which may persist for months and even years.
Mechanism of GTX-101 Bioadhesive Film Formation
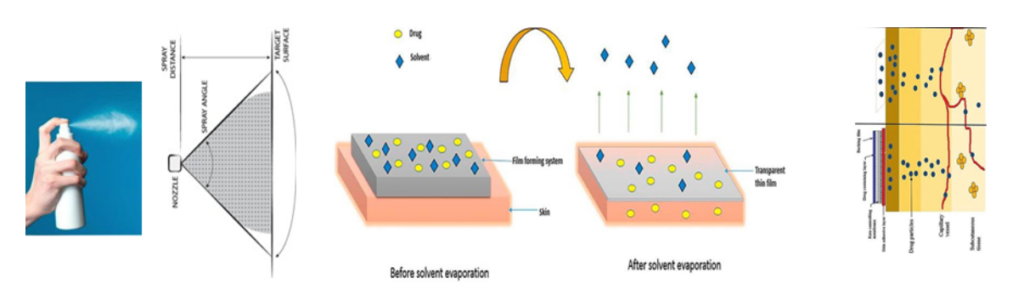
Approximately 150,000 patients a year are affected by PHN in the U.S. The total addressable market for GTX-101 is estimated to be about $400-million in the U.S. for PHN pain alone, with significant market potential in Europe and Asia.
Ms. D’Alvise says treatment of PHN most often consists of gabapentin and opioids, which can have significant side effects and are prone to abuse; and more expensive lidocaine patches, which can be hard to place and provide insufficient pain relief.
Phase 1 Single Dose PK Data in Humans
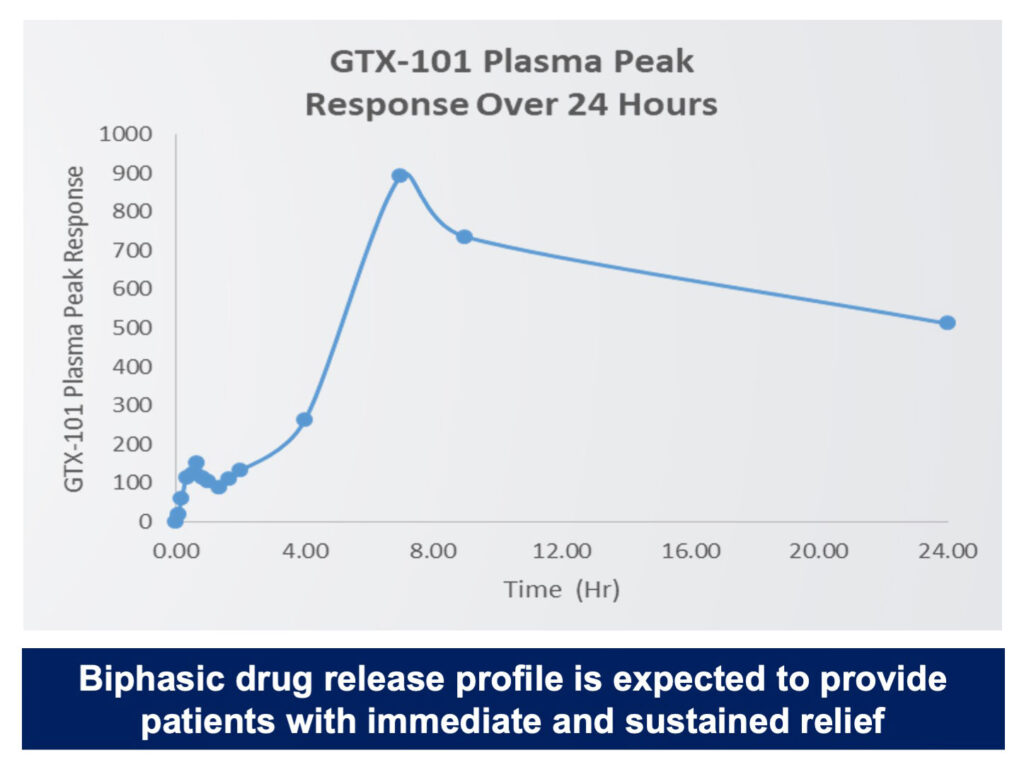
She says a metered-dose of GTX-101 spray demonstrated rapid onset of action and continuous pain relief for up to eight hours in a Phase 1 single-dose pharmacokinetic study in humans. No skin sensitivity was reported.
A single-ascending and multiple-ascending dose study with GTX-101 is expected to start in mid-2022, with results anticipated in the second half of the year. A Phase 2 trial will be required, assuming the pharmacokinetic bridging studies for GTX-104 and GTX-102 meet their endpoints, Ms. D’Alvise suggests.
“Bupivacaine is an ideal topical analgesic and has strong support from key opinion leaders because it is efficacious and opioid-sparing,” she says, adding that GTX-101 also has a “potential future market for non-PHN pain indications.”
• • • • •
To connect with Acasti Pharma or any of the other companies featured on BioTuesdays, send us an email at [email protected].






