UNITY Biotechnology’s (NASDAQ:UBX) current focus is on developing medicines to selectively eliminate or modulate senescent cells and to provide transformative benefit in age-related ophthalmologic and neurologic diseases.
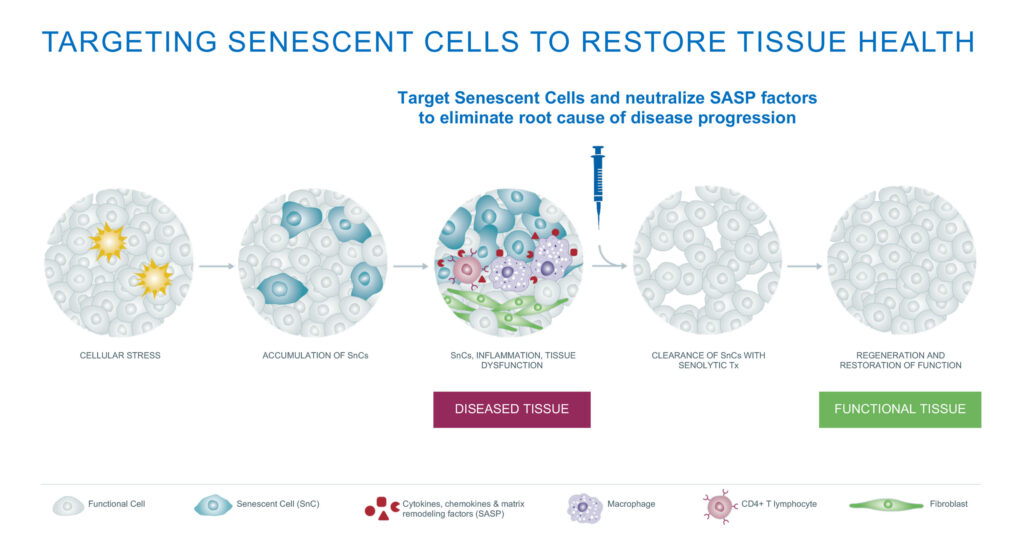
“With age, senescent cells can impact their surrounding tissue microenvironment by secreting detrimental inflammatory factors, growth factors, proteases, and fibrotic factors, representing the senescence associated secretory phenotype (SASP), which can drive disease progression,” Anirvan Ghosh, CEO of UNITY, says in an interview with BioTuesdays.
In the eye, for example, these senescent cells accumulate in blood vessels, where they can release inflammatory factors and cause blood vessels to become leaky as we age. “By targeting senescent cells, we aim to restore healthy tissue,” he says, adding that the company’s lead drug candidate, UBX1325, is the “first senolytic medicine to be evaluated in an ophthalmological clinical study.”
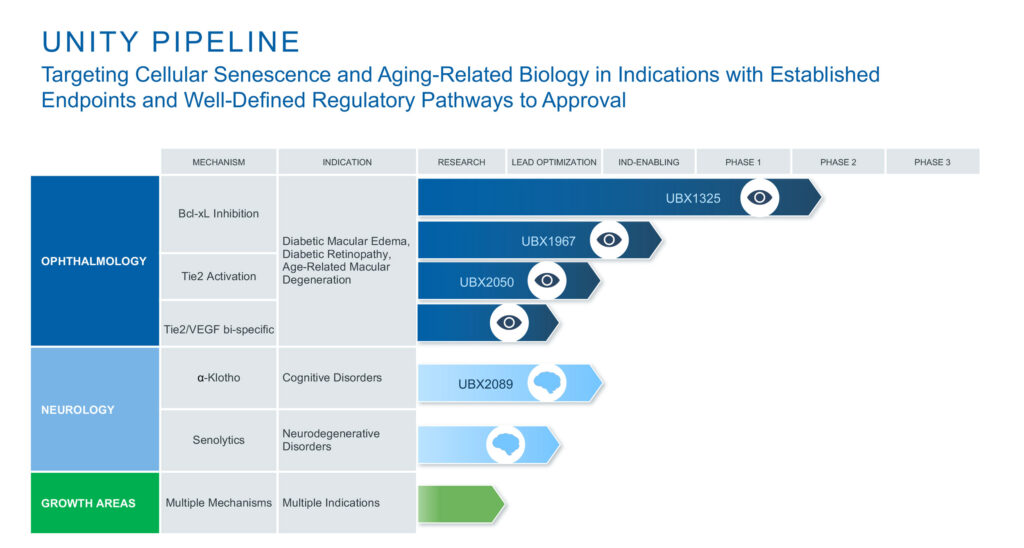
UBX1325 is being studied for age-related diseases of the eye, including diabetic macular edema, age-related macular degeneration and diabetic retinopathy. UBX1325 is a potent small molecule inhibitor of Bcl-xL, a member of the Bcl-2 family of apoptosis regulating proteins.
Mr. Ghosh explains that UBX1325 is designed to inhibit the function of proteins that senescent cells rely on for survival. In preclinical studies, UNITY demonstrated that targeting Bcl-xL with UBX1325 preferentially eliminated senescent cells from diseased tissue while sparing cells in healthy tissue, and reduced disease-related pathology.
“Our goal with UBX1325 is to transformationally improve real-world outcomes for patients with diabetic retinopathy, age-related macular degeneration and diabetic macular edema.
In July 2021, UNITY announced positive data from its Phase 1 safety study of UBX1325 in patients with advanced disease from diabetic macular edema and wet age-related macular degeneration for whom anti-VEGF therapy was no longer considered beneficial. UBX1325 was well-tolerated with no treatment-related adverse events or dose-limiting toxicities.
In addition, Mr. Ghosh says the majority of diabetic macular edema and wet age-related macular degeneration patients treated with a single injection of UBX1325 demonstrated rapid improvements in best-corrected visual acuity, central subfield thickness, and sub- and intra-retinal fluid, all key clinical measures of disease progression.
“Senolytics represent a potential new class of medicine designed to selectively target diseased blood vessels to reduce vascular leakage, allow healthy vascular remodeling, and restore retinal function,” Mr. Ghosh contends.
“We find the data we have seen to date very promising and look forward to evaluating both the safety and efficacy of UBX1325 in advanced clinical studies.”
In June 2021, UNITY dosed the first patient in a Phase 2a clinical study to assess the safety and efficacy of UBX1325 in a broader population of patients with diabetic macular edema, and data is expected in the first half of 2022.
In addition, UNITY is enrolling additional patients with advanced wet age-related macular degeneration in the Phase 1 study to gather additional data to support a Phase 2a study in wet age-related macular degeneration.
“These studies are expected to generate data to inform the efficacy of UBX1325 in a wider range of patient populations, including those who are refractory to anti-VEGF treatment,” he adds.
For more than a decade, anti-VEGF injections, such as Lucentis, Avastin and Eylea, have been leading treatments for ocular diseases, including age-related macular degeneration. “Our mechanism of action is independent of anti-VEGF, so we have the potential to be used as a monotherapy or in combination with anti-VEGF products,” Mr. Ghosh points out.
However, anti-VEGF treatments have limitations. On one hand, 15% to 30% of patients do not respond well to anti-VEGF injections, creating a large underserved population. In addition, Mr. Ghosh says UBX1325 data has been generated with only one ocular injection, compared with the monthly and bimonthly regime with anti-VEGF injections.
UNITY’s ongoing Phase 2a proof-of-concept study will evaluate the safety, efficacy, and durability of a single intravitreal injection of UBX1325 in a broader population of patients with diabetic macular edema. Approximately 60 patients who have had at least three anti-VEGF injections in the preceding six-month period will be enrolled in the study, randomized evenly between UBX1325 and sham-injected patients, and followed for 24 weeks post-injection. There will be an interim analysis of efficacy at 12 weeks.
The endpoints being explored in the study include safety and tolerability, improvements in best corrected visual acuity, retinal thickness, subretinal fluid, and durability of effect.
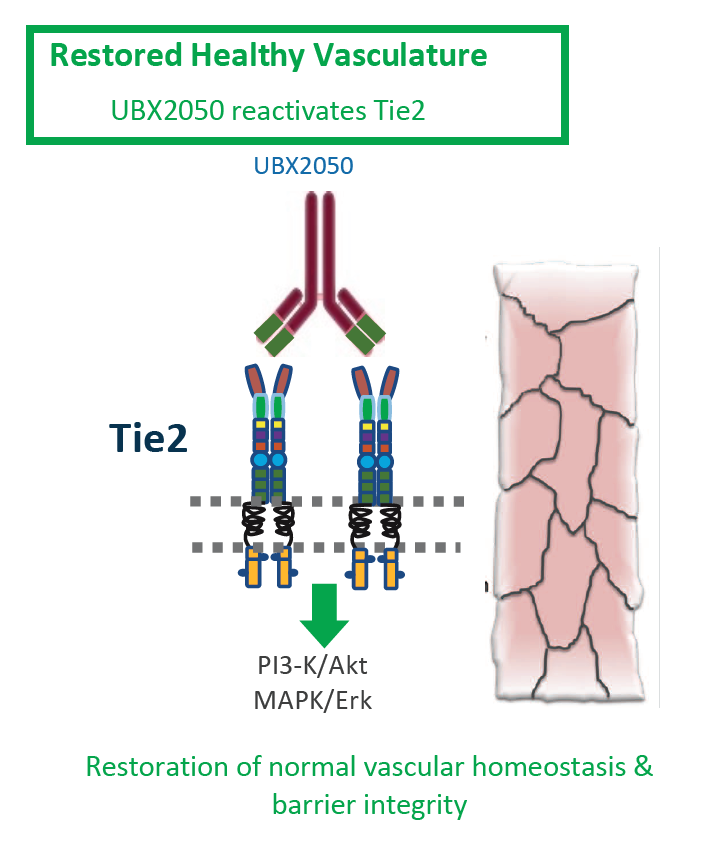
In addition to its lead Bcl-xL inhibition platform with UBX1325, UNITY has expanded its ophthalmology program with UBX2050, a Tie-2 agonistic antibody to drive Tie2 activation and potentially restore vascular function in diabetic macular edema, age-related macular degeneration and diabetic retinopathy.
UBX2050 is currently undergoing lead optimization, with IND-enabling advancement expected in 2022. Tie2 activation and the restoration of ocular tissue signaling has the potential to specifically benefit decreased vascular leakage; reduced levels of angiogenesis pathology; and blood vessel restoration in ischemic retinal regions.
Industry reports suggest there are more than one million symptomatic patients in each of the diabetic macular edema, age-related macular degeneration and diabetic retinopathy indications in the U.S. The U.S. prevalence for all three disorders is more than 20 million, with age-related macular degeneration making up the lion’s share.
Beyond the retina, UNITY is advancing its cellular senescence approach to evaluate the pathway for central nervous system inflammation reduction and potentially reverse Tau pathology.
• • • • •
To connect with UNITY or any of the other companies featured on BioTuesdays, send us an email at [email protected].