
As CEO of closely-held GeneTether, Har Grover is tackling the emerging potential of gene editing, which has come alive in the past few years. As an entrepreneur, investor, senior executive and strategist with over 30 years of experience in healthcare, life sciences and technology, Mr. Grover has worked with growth companies at all stages, from start-up to commercialization, to going public and growing through the $1-billion revenue mark. He has helped companies enter new markets both in Canada and internationally – organically and through M&A, strategic alliances, and joint ventures. Mr. Grover has been involved in a couple of earlier ventures in sectors related to gene editing, such as proteomics in the early 2000’s after the Human Genome Project mapped the human genome and identified the underlying genetic factors of many diseases, followed by exploration of which proteins can affect genes, and in stem cells and regenerative medicine. In this interview with BioTuesdays, Mr. Grover discusses what GeneTether brings to the sector.
Let’s begin with a brief history of GeneTether.
The company was formed in San Francisco in 2018 by Geoffrey Sargent, Ph.D., a biochemist and our chief scientific officer, who has been working in the field of gene editing in mammalian cells since 1987, and William Garner, M.D., an investor and entrepreneur, who has founded and co-founded numerous life science companies. Dr Garner, through his merchant bank, EGB Ventures, has had some significant success in oncology and other areas internationally. At GeneTether, although we are relatively small, we are building the company with an international footprint, with management and scientific personnel in the Bay Area, Toronto and Barcelona, and external collaborators at UC Davis, Barcelona and Germany. And we are exploring further collaborations in North America, Europe and Australia.
What has been the main focus of your early development?
For the first two years, our focus was developing a data package to protect GeneTether’s IP around our donor DNA Template-Nuclease-Tethering (TNT) platform. We received our first patent in Australia in February 2021 and have patents pending in eight other jurisdictions. The U.S. patent application is expected to be reviewed this summer. I joined the company as CEO in February 2021.
What is gene editing?
Basically, for genetic diseases where there is no cure, the objective of gene editing is to repair a mutated gene by replacing its mutated DNA with normal DNA and potentially curing the disease. Gene insertion and gene correction require delivery of a donor DNA template to the site of a DNA break. If a donor DNA template is not located near a break in the genome, correct repair will rarely incorporate the donor DNA template. The result is error-prone repair, leading to low gene editing efficiency, DNA mutations and rearrangements, and cell death. GeneTether has developed a proprietary method to conjugate, or tether, donor DNA templates to gene editing nucleases, utilizing well characterized DNA binding domains.
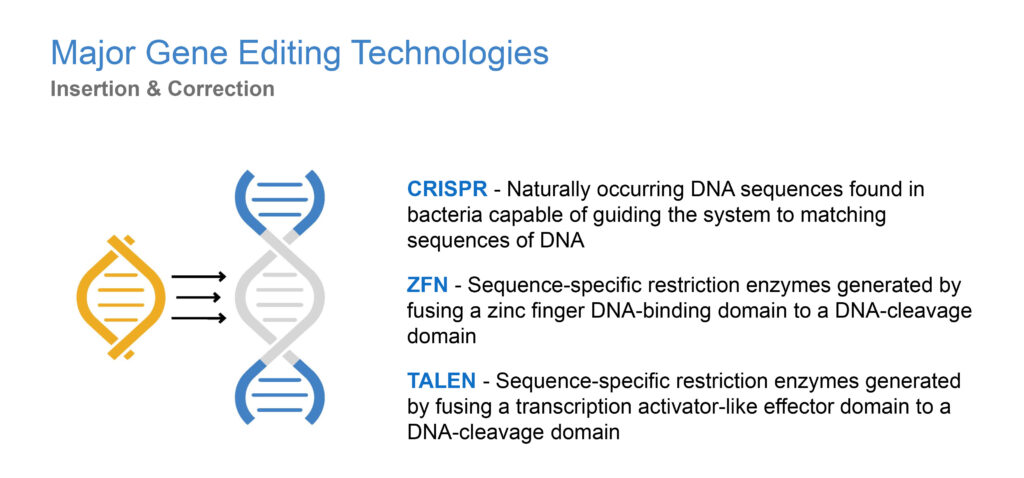
What’s a good analogy to describe gene editing?
I like to compare gene editing technologies to the way a word processor works: there is a search function, a cut function and then a repair or replace function. While there are different components that carry out the three functions, GeneTether’s platform drives the repair/replace function in a manner analogous to a word processing system being presented with the letters that need to be inserted into an empty slot rather than leaving it to the computer’s autofill function. The first gene editing technologies were developed in the late 1980s. More recently, a new gene editing tool called CRISPR, discovered in 2011, has made it easier to edit DNA.
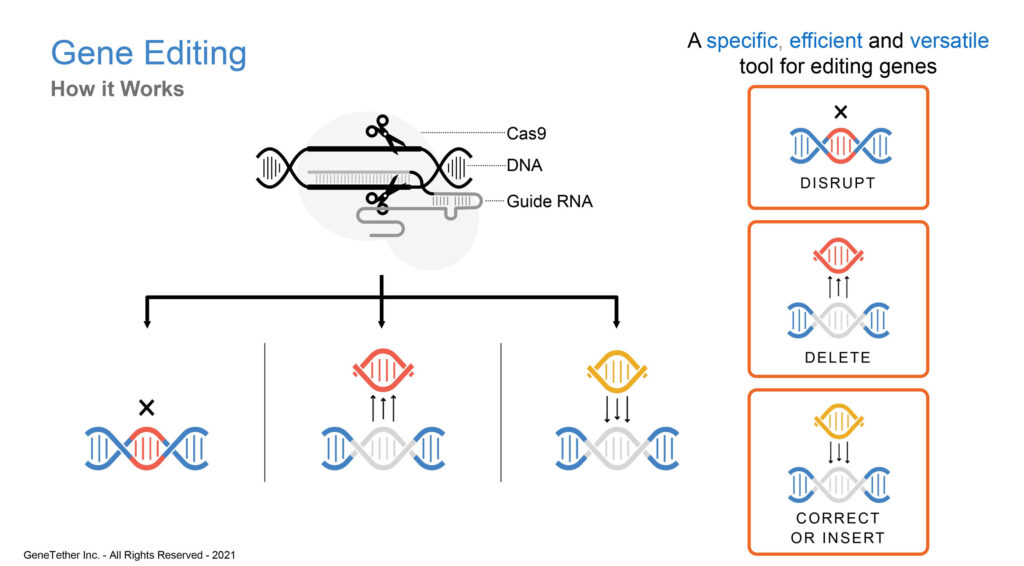
What is CRISPR/Cas9 and why is it significant?
The CRISPR/Cas9 enzyme for gene editing, has been a major technological breakthrough that enables geneticists and medical researchers to edit parts of the genome by removing, adding or altering sections of the DNA sequence. It has made genome modification in cells or organisms faster, more efficient, and more robust than previous genome editing methods. CRISPR/Cas9 does a good job of finding and cutting target DNA strands but current insertion and correction technologies are inherently error prone and inefficient. This is where we come in with our TNT platform.
What’s your solution?
Our donor DNA Template-Nuclease-Tethering technology is a powerful next generation CRISPR platform technology. The path to taking our gene editing tool and developing a therapeutic requires fully validating the platform by rounding out our data package in different cell lines and editing scenarios, while still maintaining a high degree of efficiency. Then, we have to more fully test it within a disease target to obtain in vivo data and lastly, as the therapy gets fully developed, delivery into the target cell needs to be addressed using either viral vectors or other non-viral vectors for delivery to show a therapeutic benefit in a clinical trial setting. While we have a fair bit of work to do, we believe our technology platform is a game changer and can enable gene editing to advance to the next stage.
Can you explain what you mean by tethering in your platform?
There are two ways you can help a gene repair itself using CRISPR/Cas9: leave it on its own and hope it will repair itself. This would be using the non-homologous end joining method and is very error prone. The second pathway is homology directed repair, whereby a donor DNA template, with a properly formed DNA strand is inserted into the missing gene slot. Our secret sauce involves dragging a DNA template to the site of the CRISPR/Cas9 cutting, by tethering CRISPR/Cas9 with the donor template, all at the same time, so the donor template is nearby and not just floating around the cell.
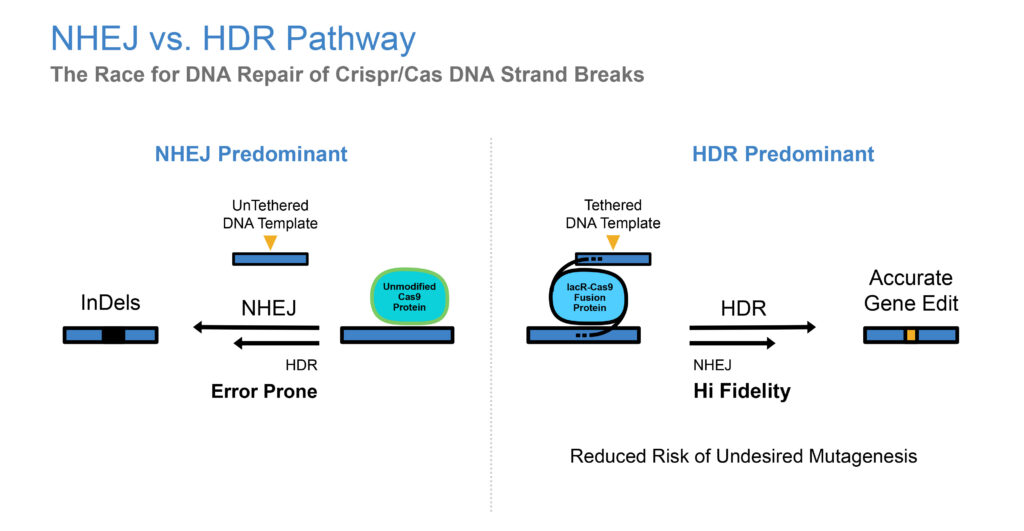
What sort of results have you achieved with the TNT platform?
Our initial data shows a sevenfold increase in editing efficiency, which is defined as the number of potential gene mutations you can target versus the number that get completed. Typical gene editing efficiency has been between 0.5% and 20%. However, the general view is that to get an effective therapeutic, you need more than 50% editing efficiency.
What are your plans going forward?
We’ve raised around $1-million from family and friends and are contemplating a $10-million raise towards the end of 2021. The financing would likely be in connection with a go-public arrangement on a Canadian exchange, which will be determined soon.
*Editor’s Note: This article does not constitute an offer to sell or the solicitation of an offer to buy any securities of GeneTether, and shall not constitute an offer, solicitation or sale of any security in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction.
• • • • •
To connect with GeneTether or any of the other companies featured on BioTuesdays, send us an email at [email protected].






