As founder and executive chair of the non-profit Indo-US Organization for Rare Disease (IndoUSrare), Harsha Rajasimha, Ph.D., social entrepreneur, and subject matter expert in bioinformatics, data analysis, and eClinical software development, is passionate about accelerating orphan drug development and removing disparities and inefficiencies in rare disease clinical trials.
IndoUSrare, an independent non-profit organization, is focused on patients with rare diseases of Indian origin living in the USA, India, or globally. The humanitarian charitable organization aims to build collaborative bridges between the East and West to accelerate research and development of diagnostics and therapies through education, advocacy, research, and grants.
In this interview with BioTuesdays, Dr. Rajasimha touches on the need for cross-border collaborations to address disparities in rare disease clinical trials, the acceleration of the research and development of rare disease orphan drugs and gene therapies, and the new FDA pilot program, START.
Let’s begin with a brief history of your connection to rare diseases.
With a background in computer science, I completed my doctoral research in the interdisciplinary program in genetics, bioinformatics, and computational biology right around the time that the first human genome was sequenced in the early 2000s. The promise and potential of diagnosing and treating genetic diseases was enormous and it was very exciting to be at the forefront of the precision medicine and genomic revolution.
In 2012, my wife and I experienced a personal connection to rare disease when our daughter was born with Edwards Syndrome or Trisomy 18, a rare chromosomal condition. Suddenly, I was a rare Dad. I connected with the NIH Office of Rare Disease Research and as I became more involved it was very clear that while significant progress in rare disease research had occurred in the U.S. and European Union following the 1983 Orphan Drug Act, 90% of the global population continued to live in regions with no rare disease policy or regulation. Furthermore, I discovered a diversity gap with huge disparities in race, ethnicity, age, and gender of participants in rare disease clinical trials. This was all very eye-opening for me.
Did identifying these disparities inspire you to form IndoUSrare?
Yes. We need to build trust with underrepresented populations not just from a race and ethnicity standpoint but age and gender as the majority of clinical trial participants tend to be adult males from affluent backgrounds.
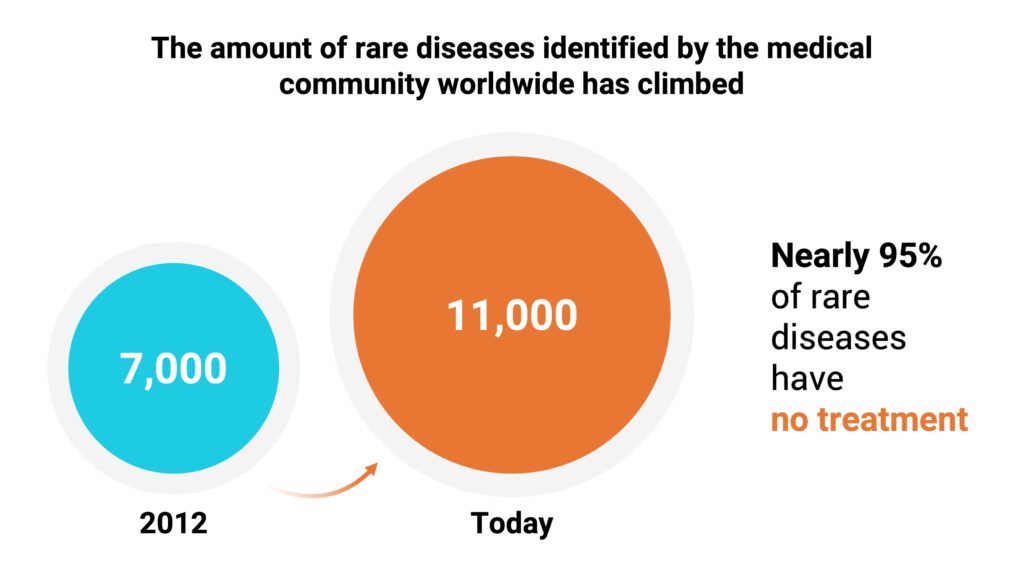
The disparities in race and ethnicity in rare disease clinical trials have universal health impacts in that there are significant gaps in understanding diseases and conditions along with the economic burden of healthcare. Since 2012, the amount of rare diseases identified by the medical community has climbed from 7,000 to 11,000 worldwide. Nearly 95% of rare diseases have no treatment.
The U.S. has the world’s most forward-looking regulatory framework for innovative new medicines including cell and gene therapy. In 2019, I founded IndoUSrare as a gateway to connect the U.S. ecosystem of orphan drug development with South Asia. Genetically diverse and the most densely populated region of the world, it accounts for a quarter of the world’s population but only 2% of clinical trial participants globally.
My focus with IndoUSrare is on education, training, and identifying ways to include this important segment of the world’s population in cross-border research collaborations. Sharing resources helps to bridge gaps in understanding diseases and conditions which benefits all patients and clinical stakeholders.
How does IndoUSrare assist patients and families affected by rare diseases?
IndoUSrare acknowledges the important role that parents, caregivers, and patient advocacy groups provide in moving the needle on the orphan drug development process. So, we have formed a very successful Patient Concierge Program and Patient Alliance Program as a way to connect families and patient advocacy groups throughout the U.S. and India with each other and with industry partners and collaborators such as doctors, diagnostic facilities, patient assistance programs, insurance programs, and financial resources. This program has enabled the creation of a data-rich patient and rare disease database which is invaluable in the rare disease space. IndoUSrare has also formed the Corporate Alliance Program which plays a critical role in bringing companies involved in genetic disease diagnosis, drug development, biotech, and pharmaceutical together and accessible to our patient advocacy groups.
What is your role in accelerating orphan drugs and gene therapy R&D?
The process of developing new drugs, not just for rare diseases but for all diseases, has been historically inefficient. In fact, over the last several decades it has not improved but has actually been deteriorating.
We know that medical breakthroughs are reliant on better clinical trials. So, at the same time that I established IndoUSrare, I founded Virginia-based start-up, Jeeva Informatics Solutions, a unified e-clinical software platform that aims to boost clinical trial operations by reducing costs and speeding up drug discovery and development through technology, AI automation, and user-friendly software. Jeeva is a patient-centered design guided by stakeholder needs and regulatory requirements.
By digitizing and automating manual repetitive tasks and reducing the logistical burdens on patient and study teams by more than 70%, we are accelerating the clinical research process of bringing new medicines or vaccines to patients who need them – three times faster. Our platform is fully scalable and facilitates diverse patient enrollment, engagement, and evidence generation on any browser-enabled mobile device. Jeeva enables the highly efficient execution of clinical trials with features such as bi-directional communications, eligibility screening, scheduling, and remote, touchless electronic informed consent. This one-stop platform is especially helpful for orphan drugs with modest resources.
Together, IndoUSrare and Jeeva are fully addressing critical gaps and inequities by connecting stakeholders between the U.S. and the Indian subcontinent at all levels including regulatory, industry, patient advocacy, and medical, genetic, and physician researchers.
It has been a beautiful journey so far.
Tell us about the FDA pilot program START.
This past October, we held the inaugural Indo US Bridging RARE Summit at George Mason University in Arlington, Virginia to raise awareness and support for the more than 30 million Americans and 70 million Indians affected by rare diseases. It was a massive success.
Our keynote speaker was Peter Marks, MD, Ph.D., Director of the Center for Biologics Evaluation and Research at the FDA. At the Summit, Dr. Marks announced the launch of the Support for Clinical Trials Advancing Rare Disease Therapeutics, or START program, designed to expedite the development of new drugs and biological products for rare diseases. This initiative aims to increase communication between the FDA, international regulators, and sponsors to address specific development issues and offer ways to enhance program development and generate reliable data for regulatory submissions.
It’s essentially Operation Warp Speed for gene therapy. If we can create a Covid-19 vaccine in nine months, why not an orphan drug in nine months? Developing and marketing a standard drug can take up to 12 years with a significant amount of time spent in clinical trials. Now that we have seen what global cooperation can achieve, patients who are desperately waiting for orphan drugs are no longer willing to accept the speed or costs of the past. If the FDA’s Operation Warp Speed 2.0 and the START program are successful, it will help in exploring the possibility of concurrent collaborative review of applications with global regulatory partners.
Moreover, by leveraging AI, we will be able to accelerate rare disease research. However, to address AI bias, we need to urgently establish patient registries, conduct natural history studies, and digitize rare disease data in diverse countries such as India.
Final thoughts…
There was a high level of interest among Indo US Bridging RARE Summit 2023 speakers and attendees in working together toward a blueprint for U.S.—India cooperation. The last 40 years, since the Orphan Drug Act, have been great in delivering 1,100 FDA-approved orphan drugs but there are 11,000 known rare diseases, so we have a long way to go. Cooperation between these two countries, in alignment with the FDA’s START program and Operation Warp Speed 2.0, can potentially turbocharge the discovery and development of new drugs and biological products for rare diseases over the next 40 years. And that’s something we are very excited about.
• • • • •
To connect with IndoUSrare or any other companies featured on BioTuesdays, send us an email at [email protected].