Personalis (NASDAQ:PSNL) is developing a liquid biopsy assay designed to detect minimal residual disease (MRD) and cancer recurrence earlier than other diagnostic methods.
“Patients’ cancer journeys are filled with uncertainty, with patients and their loved ones wondering how serious the cancer is, whether it is a high- or low-risk cancer, and whether or not their treatment is working,” Chris Hall, Personalis’ president and CEO, says in an interview with BioTuesdays.
“And, after treatment, they are constantly worrying about whether the cancer is gone, and if it is coming back. We believe we have the most sensitive MRD-based test that will provide the most value to patients, their families, and their health care team,” he adds.
Mr. Hall says that imaging – including x-ray, CT, MRI and ultrasound – is the main method of determining cancer status. “The downside of imaging is that it is not always specific and/or sensitive. Imaging is also costly, with some methods exposing patients to radiation.”
Personalis’ NeXT Personal is a tumor-informed liquid biopsy assay that Mr. Hall says is 10-to-100 times more sensitive than other MRD tests. A tumor sample is taken from the patient to create a personalized genetic fingerprint of the cancer. Patients’ blood samples are then screened for the corresponding circulating tumor DNA (ctDNA) at baseline and at regular intervals.
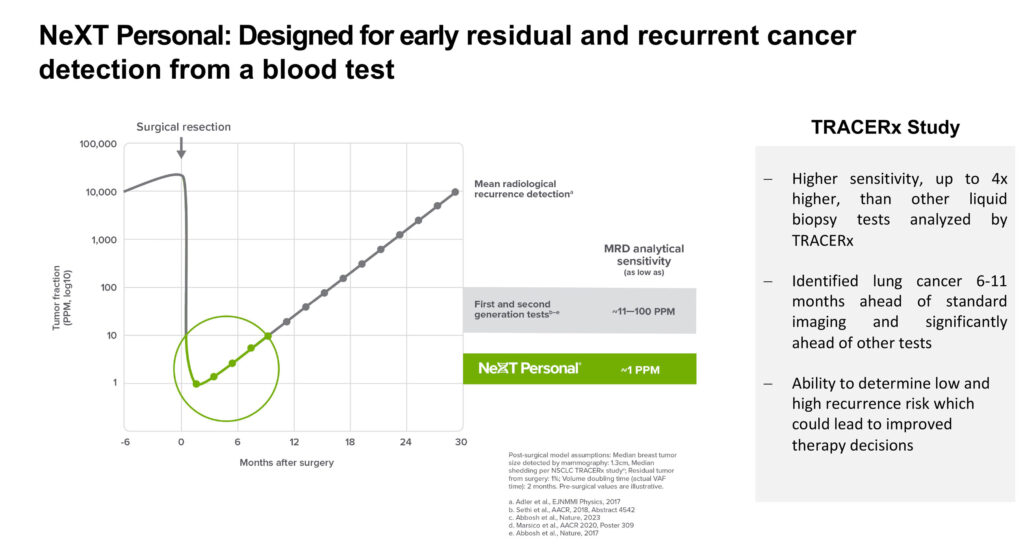
“NeXT Personal has a one-part-per-million sensitivity, which enables the earlier detection of cancer recurrence and the proactive initiation of therapy. On the other hand, should the test detect no cancer, patients may be able to avoid unnecessary treatment,” he points out.
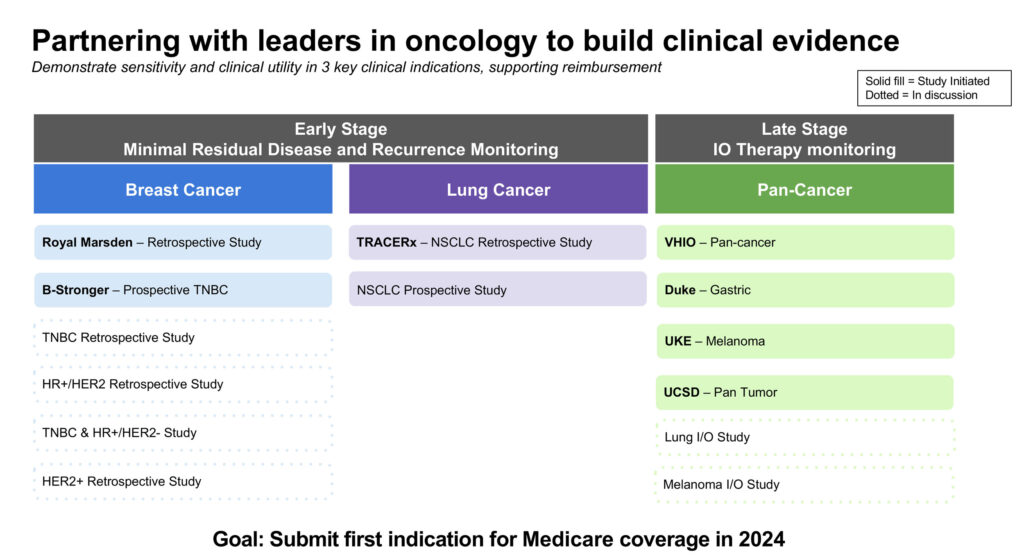
The company is focusing on three indications for NeXT Personal: early-stage lung and breast cancer, and for monitoring late-stage cancer patients who are receiving immunotherapy. “We chose to focus on early-stage lung and breast cancer because cells from these cancers aren’t readily shed into the bloodstream,” he explains, adding that these cancers present a good opportunity to validate NeXT Personal’s sensitivity.
Mr. Hall says that Personalis is pursuing a partner-centric model to scale more rapidly as it eyes what it sees as a $20-billion market opportunity.
In April 2023, Personalis announced its partnership with Cancer Research UK’s Cancer Research Horizons, University College London, and the Francis Crick Institute for the TRACERx study, an ongoing longitudinal study tracking the evolution of early-stage non-small cell lung cancer (NSCLC).
The company recently announced that NeXT Personal significantly improved detection rates for early-stage lung cancer, including lung adenocarcinoma (LUAD), a common type of NSCLC that is particularly challenging to identify in the blood.
NeXT Personal demonstrated 100% sensitivity for ctDNA in pre-surgical non-LUAD samples and 81% sensitivity for ctDNA in pre-surgical LUAD samples. The study showed that pre-surgical ctDNA levels detected with the assay could be used to classify early-stage lung cancer patients into lower- and higher-recurrence risk groups.
In addition, the study demonstrated that NeXT Personal enabled earlier detection of residual or recurrent lung cancer after surgery, with a median lead time of some six-to-11 months ahead of traditional imaging.
Personalis is also collaborating with the Academic Breast Cancer Consortium (ABRCC) and Criterium on a prospective clinical trial that is evaluating NeXT Personal’s ability to detect MRD during and after treatment, and recurrent cancer in patients with early-stage, resectable, triple-negative breast cancer.
For the late-stage immunotherapy monitoring indication, Personalis is testing NeXT Personal in four studies. It is collaborating with Duke University in a gastric cancer study, the University Cancer Center Hamburg on a melanoma study; and the Vall d’Hebron Institute of Oncology and the University of California San Diego on pan-cancer studies.
“In patients who are receiving immunotherapy, tumor size isn’t always an indicator of treatment efficacy,” Mr. Hall says. “We cannot solely rely on imaging because some tumors grow bigger even though the therapy is working. And unfortunately, immunotherapy is not always effective, so determining which patients are not improving can save them the unnecessary treatment.”
Personalis launched in October NeXT Personal Dx via an early access program. “Our goal is to build clinical evidence and submit an application for Medicare coverage in 2024 for our first indication,” he notes.
The company has applied NeXT Personal in numerous partnerships with biopharma and biotech companies. “We’ve collaborated with 16 of the top 20 pharma companies and 15 immuno-oncology companies to help them screen for patients that are more likely to respond to treatment, as well as for monitoring the effects of their immunotherapy,” Mr. Hall points out.
Personalis has also helped 18 companies develop personalized cancer vaccines. The company’s ImmunoID NeXT is a biomarker discovery program that conducts exome and transcriptome sequencing and computational analysis to predict and select cancer vaccine neoantigens.
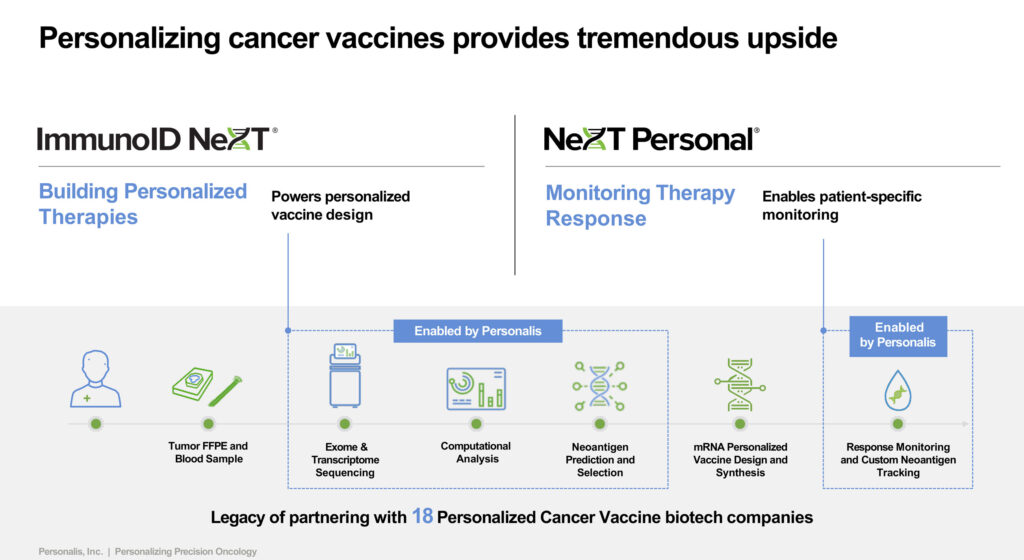
In December 2022, Moderna and Merck announced that their investigational personalized mRNA cancer vaccine for stage 3/4 melanoma, in combination with pembrolizumab, met its primary efficacy endpoint in Phase 2b trial.
“We are proud to be the engine behind Moderna and Merck’s vaccine and expect our partnership with Moderna to accelerate our revenue over the next 12-to-24 months,” he says.
• • • • •
To connect with Personalis or any other companies featured on BioTuesdays, send us an email at [email protected].