
Aravive (NASDAQ:ARAV) is taking a differentiated approach with its lead molecule, batiraxcept, to target inhibition of the GAS6/AXL signaling pathway in several cancer indications.
“Activation of the GAS6/AXL signaling pathway causes tumor invasion and metastasis, and high levels of GAS6 and AXL have been strongly associated with drug resistance, rapid disease progression, and lower survival,” Gail McIntyre, Ph.D., CEO of Aravive, says in an interview with BioTuesdays.
Dr. McIntyre says increased expression and signaling of GAS6 and its main receptor, AXL, are seen in many cancers and are correlated with a poor prognosis. “There is extensive literature on GAS6/AXL involvement in metastatic disease. This is a challenging target because of the high affinity between GAS6 and AXL,” she adds.
Dr. McIntyre explains that industry attempts to target the GAS6/AXL pathway have fallen short, causing either undesirable off-target effects or limited efficacy. “To circumvent these challenges, we are taking the entirely novel approach of targeting the GAS6 ligand, rather than the AXL protein. To our knowledge, we are the only company targeting GAS6.”
She says batiraxcept is an ultra-high affinity decoy protein that binds to GAS6, the sole ligand that activates AXL, thereby inhibiting metastasis and tumor growth, and restores sensitivity to anti-cancer agents.
Batiraxcept (AVB-500) Inhibits Metastasis and Tumor Growth
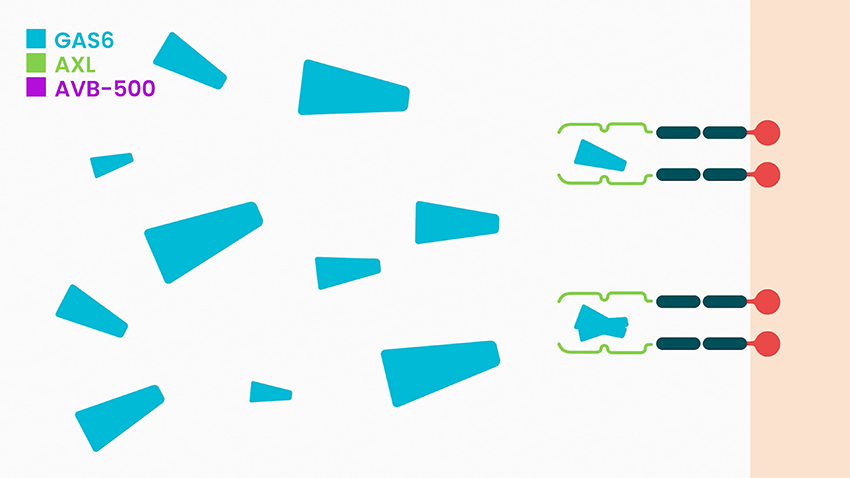
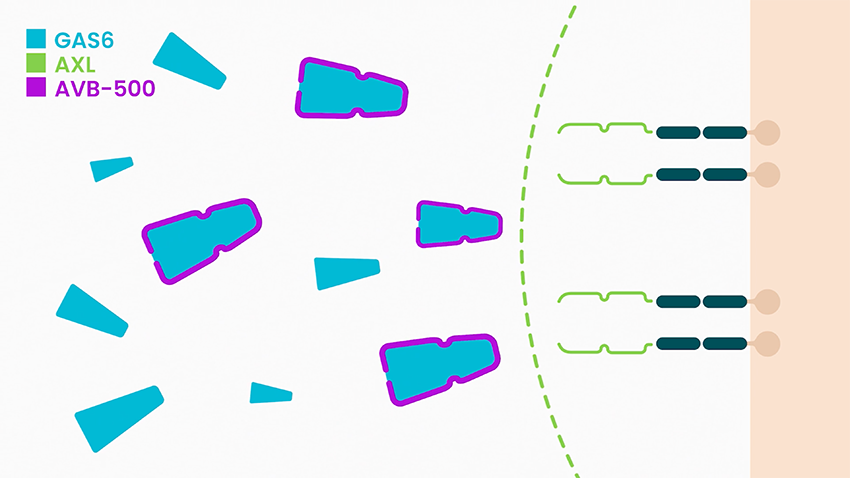
In clinical studies to date, Dr. McIntyre says batiraxcept has demonstrated a favorable safety profile and compelling early clinical activity in cancer patients, suggesting it could be “ideal for use in combination with many anti-cancer drugs or as a maintenance therapy.”
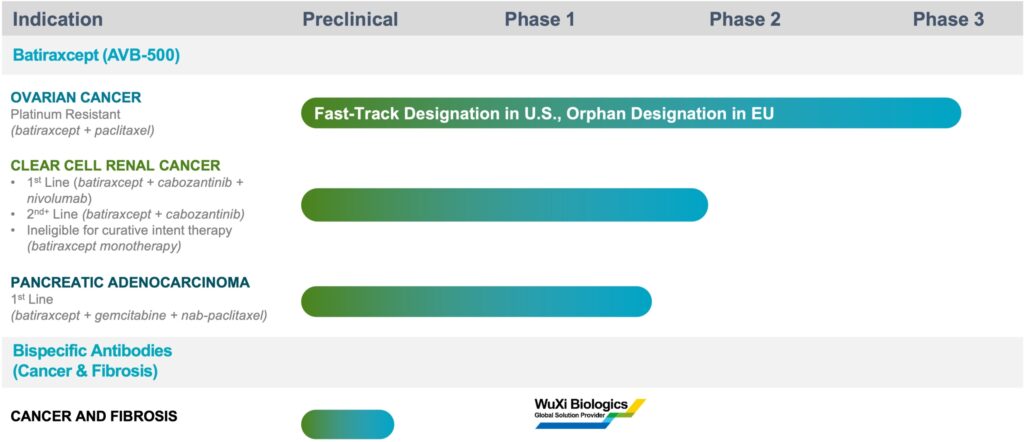
Batiraxcept is in an active registrational Phase 3 trial in platinum resistant ovarian cancer in combination with standard of care, paclitaxel; a Phase 1b/2 trial in clear cell renal cell carcinoma in combination with carbozantinib; and a Phase 1b/2 trial in pancreatic adenocarcinoma in combination with gemcitabine and nab-paclitaxel.
The company used modeling to identify a dose of 15 mg/kg of batiraxcept as the appropriate dose to take into the Phase 3 study.
Dr. McIntyre points out that the Phase 1b platinum-resistant ovarian cancer trial demonstrated more than a doubling of progression-free survival than standard of care with paclitaxel in a patient subgroup that represents the ongoing Phase 3 population.
In November, Aravive released preliminary data from the Phase 1b portion of its open-label Phase 1b/2 renal cell carcinoma trial, which showed that seven of 16, or 44%, of patients receiving batiraxcept in combination with carbozantinib achieved the best overall response rate. Cabozantinib alone produces response rates between 17% and 28% in a similar population, she adds.
The Phase 1b data also demonstrated that 14 of 16 patients, or 88%, had tumor shrinkage and five of seven patients with a confirmed response had at least 16 weeks of follow up.
“These results reinforce our confidence in the potential of batiraxcept to improve outcomes across multiple types of cancer by targeting the GAS6/AXL signaling pathway,” Dr. McIntyre contends.
In August, Aravive dosed the first patient in the Phase 1b portion of its Phase 1b/2 trial of batiraxcept in combination with gemcitabine and nab-paclitaxel as a first-line treatment in patients with advanced or metastatic pancreatic adenocarcinoma, one of the most difficult-to-treat cancers with a high mortality rate.
The randomized, controlled Phase 2 portion of the pancreatic cancer clinical trial is designed to evaluate batiraxcept as a first-line therapy in combination with gemcitabine and nab-paclitaxel versus gemcitabine and nab-paclitaxel alone. The primary endpoint of the Phase 2 portion of the trial is progression-free survival. The secondary endpoints are objective response rate, duration of response, clinical benefit rate, safety and overall survival, and the exploratory endpoints are pharmacokinetics and pharmacodynamics.
“And we have a wealth of preclinical data suggesting we could go into a broad range of cancer indications in solid metastatic tumors and leukemia, as well as in earlier-stage patient populations,” she adds.
Batiraxcept has been granted fast track designation by the FDA and orphan drug designation by the European Commission in platinum-resistant recurrent ovarian cancer.
Aravive’s IP protection includes 28 issued patents in the U.S. and globally as well as five patents pending that expire between 2031 and 2042.
Further validation of batiraxcept comes from an exclusive outlicensing partnership with 3D Medicines for development and commercialization of batiraxcept across all oncology indications in Greater China. Aravive has received $21-million thus far from this agreement, including an upfront payment of $12-million, and is eligible for $207-million in milestone payments and additional tiered royalties on annual net sales.
The company also is working with WuXi Biologics, a Chinese contract development and manufacturing organization, to identify and develop high-affinity bispecific antibodies against connective tissue growth factor, targeting cancer/fibrosis. “This is a domain-focused strategy to potentially generate a best-in-class clinical therapeutic, targeting desmoplasia and tumor growth in 2023,” she points out.
Dr. McIntyre says Aravive expects to have a busy news flow in 2022, with initiation of the Phase 2 portion of its kidney cancer trial and updated Phase 1 and preliminary Phase 2 data in kidney cancer. In addition, the company expects to complete enrollment in its Phase 1b pancreatic cancer trial, and initiate the Phase 2 portion of the trial.
In mid-2022, she notes that Aravive plans to release an interim analysis after 85 patients have progressed in the Phase 3 ovarian cancer trial to determine whether it needs to enroll an additional 100 bevacizumab-naive patients to increase the chances for statistically significant progression free survival.
Phase 3 data will be released either in early-or mid-2023, depending on whether the study evaluates 300 or 400 patients. The company plans to file a BLA with the FDA for batiraxcept in platinum resistant ovarian cancer in the second half of 2023.
Aravive’s plans for 2023 also include release of updated Phase 1b and preliminary Phase 2 data in the pancreatic cancer trial; updated Phase 2 kidney cancer trial data; and filing of an IND in the connective tissue growth factor program.
According to Dr. McIntyre, Aravive’s three cancer indications represent large market opportunities of about 105,000 combined addressable patients a year plus the potential to expand into other cancers and earlier-stage patients.
Platinum resistant ovarian cancer, the company’s lead target indication, is the fifth leading cause of cancer deaths among women in the U.S., with about 70% of 12,000 patients diagnosed with advanced-stage disease. In metastatic clear cell renal carcinoma, its second target indication, the five-year survival rate is 12%. And, in pancreatic cancer, which is projected to be the second leading cause of cancer deaths in the U.S. by 2030, the five-year survival rate remains a dismal 11%.
“As cancer is a multi-faceted disease controlled by a number of different genetic and molecular factors, inhibiting the GAS6/AXL pathway may provide a promising anti-metastatic approach that is complementary to established cancer treatments,” Dr. McIntyre says.
• • • • •
To connect with Aravive or any of the other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.







