
POINT Biopharma Global (NASDAQ:PNT) is accelerating the development of precision medicines for the treatment of cancer, with a late- and early-stage pipeline of radiopharmaceuticals, a technology platform for pipeline expansion, and a fully vertically integrated supply chain – from rare medical isotopes to manufacturing – capable of supplying North America and Europe with radiopharmaceuticals.
“A supply chain drives everything in radiopharmaceuticals,” Joe McCann, Ph.D., co-founder and CEO of POINT, says in an interview with BioTuesdays. “Radiopharmaceuticals are made just-in-time on a daily basis, with a useable life measured in days. Manufacturing and a supply chain are key success factors in our sector.”
Cardiologists have used radiopharmaceuticals for decades, primarily as a diagnostic tool for cardiac conditions, while oncologists currently use them for both diagnostic and therapeutic purposes.
“We are focused on creating new radiopharmaceutical therapies by developing more effective targeting agents and combining them with isotopes that can be administered in outpatient settings,” Dr. McCann points out.
“This is a game changer and the next pillar of cancer treatment, taking these therapies out of specialized institutions and into outpatient settings.”
Dr. McCann explains that radiopharmaceuticals offer unique qualities that make them a precise and powerful tool for the treatment of cancer. Unlike most radiation therapies, radiopharmaceuticals are molecularly targeted, which enables a significant reduction in the overall amount of radiation a patient is exposed to.
“An opportunity exists to develop radiopharmaceuticals against multiple validated targets, and they also may be prime candidates for combination with both DNA damage response agents and checkpoint inhibitors,” he adds.
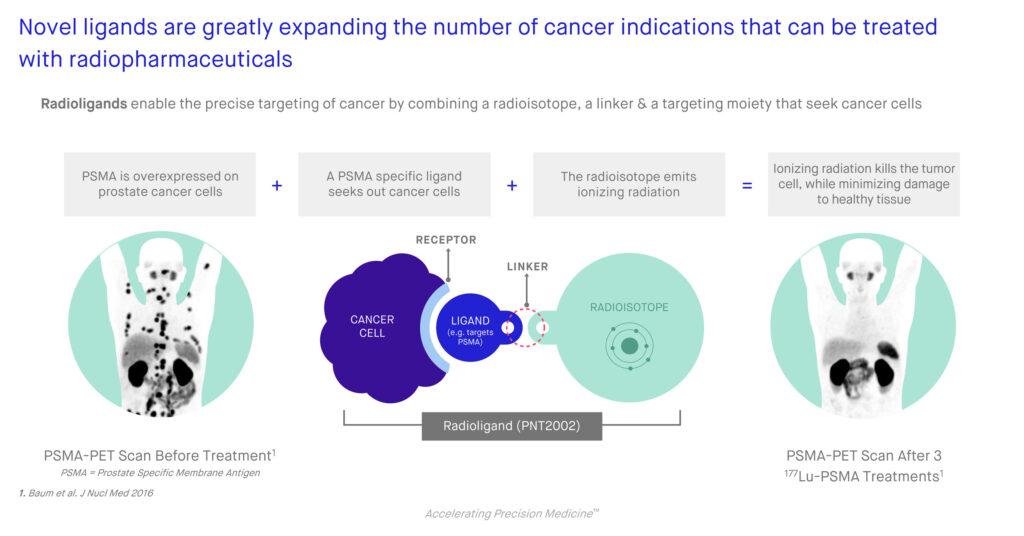
According to Dr. McCann, POINT has developed novel radioligands that include a medical isotope, a linker and a ligand that are designed to bind with specific receptors overexpressed in tumors. The linker attaches the medical isotope to the ligand, enabling the medical isotope to be delivered to the tumor. The medical isotope emits high-energy particles that damage the DNA of the cells within the tumor, leading to cell death.
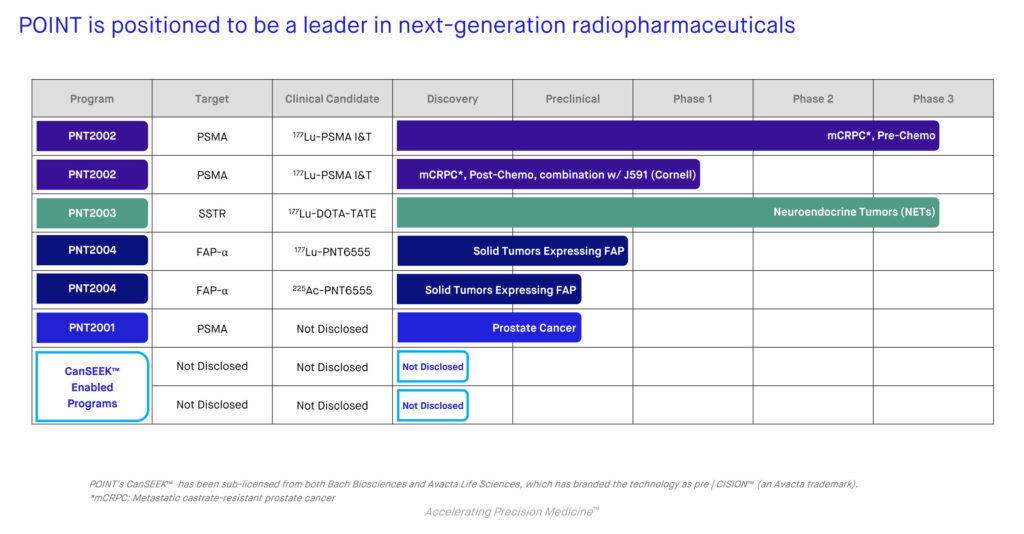
POINT has developed two late-stage assets that are designed to overcome the barriers that have prevented widespread commercialization of radioligands: PNT2002 for metastatic castration-resistant prostate cancer (mCRPC) and PNT2003 for the treatment of patients with neuroendocrine cancer.
PNT2002 is a prostate-specific membrane antigen (PSMA)-targeted therapy, which is overexpressed in more than 85% of prostate cancers, but very limited in normal tissues. PNT2003 is a somatostatin receptor (SSTR)-targeted radioligand therapy for the treatment of patients with SSTR-positive neuroendocrine cancer.
Dr. McCann says POINT is currently focused on two radioisotopes: Lutetium-177 (Lu-177), which emits small particle electrons and has a half-life of 6.6 days, and Actinium-225 (Ac-225), which emits large alpha particles, with a half-life of 10 days. POINT’s discovery-stage programs are also investigating the use of emerging radioisotopes such as Terbium-161 (Tb-161) and Astatine-211 (At-211).
Last October, POINT inked a supply agreement for the isotope Ytterbium-176 (Yb-176) with Toronto-based Kinectrics, a service provider to the nuclear power and electricity industries, to support POINT’s in-house production of no-carrier added (n.c.a.) Lu-177. Yb-176 is the input material for creating n.c.a. Lu-177, without long-lived radioactive impurities present in Lu-177 production routes that use Lu-176.
“With this agreement with Kinectrics, POINT is laying the groundwork to access large quantities of highly enriched Yb-176, creating the world’s first, stable North American supply of n.c.a. Lu-177, and establishing POINT as a dependable provider of high-quality radiopharmaceuticals,” Dr. McCann contends. “Ensuring a secure, redundant and consistent isotope supply is a cornerstone of POINT’s radiopharmaceutical platform.”
POINT also has additional supply agreements with ITM of Germany and Isotopia of Israel for n.c.a. Lu-177. Most n.c.a. Lu-177 manufacturers depend on Russia for their Yb-176 isotope supply, which is then irradiated in nuclear reactors to produce Lu-177.
Beyond Lu-177, POINT has supply agreements with TerraPower, Northstar Medical Radioisotopes and Ionetix for Ac-225. The unique feature of these supply agreements is these firms have access to rare thorium and radium, which are rare input materials used to generate Ac-225. POINT is currently developing Ac-225 radiopharmaceuticals targeting solid tumors that express prostate specific membrane antigen (PSMA) and fibroblast activation protein-alpha (FAP-alpha).
By combining different radioisotopes with each ligand and looking at these combinations in different cancer models in the development process, POINT can find the best radioisotope for its next-generation radiopharmaceuticals. POINT’s pipeline includes PNT2002, PNT2003 and early-stage PNT2004 for the treatment of tumor types expressing fibroblast activation protein-alpha, which is present in more than 90% of epithelial tumors.
The company’s 80,000-square-foot manufacturing plant in Indianapolis is licensed for alpha-and beta-emitting isotopes and contains dedicated space for commercial-scale radiopharmaceutical production and high-speed automated packaging.
Dr. McCann says shipments from the facility can reach 50% of the U.S. population within 12 hours by local ground transportation, and to the rest of North America and Western Europe within 12-to-48 hours by air.
POINT’s PNT2002 is a PSMA-targeted therapy for metastatic castration-resistant prostate cancer (mCRPC). Each year in the U.S., 12,000 men are diagnosed with mCRPC and an additional 40,000 progress from earlier stages of the disease, representing a $5.1-billion market.
The company leveraged published academic data to advance PNT2002 directly in the Phase 3 SPLASH program in the US and Canada. In an earlier clinical trial in patients with mCRPC, published academic data showed Lu-PSMA I&T, the ligand used in POINT’s PNT2002 program, demonstrated a median radiographic progression-free survival of 13.7 months and a median overall survival that was not reached at a 28-month follow-up, with no significant adverse events during early monitoring.
POINT plans to enrol approximately 400 patients across North America, Europe, and the UK in SPLASH, which commenced in early 2021 in the U.S. and Canada. Patients will be randomized 2:1 with those in arm A receiving PNT2002 and patients in arm B receiving standard of care for patients that fail second-line hormonal treatment, either abiraterone or enzalutamide, and are chemo-averse or chemo-ineligible. Patients in arm B who experience centrally-assessed radiographic progression and meet protocol eligibility will have the option to crossover and receive PNT2002.
SPLASH patients will be followed for up to five years from their first PNT2002 dose. The primary endpoint of the study is radiographic progression-free survival. Key secondary endpoints include overall response rate, overall survival, and pharmacokinetics. Topline data is expected in mid-2023.
In 2021, POINT licensed clinical data from an ongoing trial as well as unique intellectual property from CanProbe, a joint venture between the University Health Network in Toronto and the Centre for Probe Development and Commercialization in Hamilton, Ontario. CanProbe has its own formulation method for Lu-DOTA-TATE, which has been named PNT2003.
The formulation of DOTA-TATE licensed by POINT uses the medical isotope n.c.a. Lu-177, which is preferred by many clinics because it doesn’t contain any long-lived radionuclides that can complicate administration and force the creation of costly specialized waste streams, Dr. McCann points out.
Interim data from an ongoing Phase 3 clinical trial in Canada with PNT2003 has been positive. The progression-free survival rate at 12 months of 89.3% exceeded pre-specified success thresholds of greater than 60%. Full results from the trial are expected in mid-2022. POINT expects to confirm the optimal regulatory and commercial pathways for this program in the first quarter of 2022.
In addition, the company’s CanSEEK prodrug technology platform is designed to improve precision and safety of radioligands. The CanSEEK technology, currently in preclinical development, blocks a radioligand’s ability to bind to receptors until it is removed by FAP. Once the prodrug reaches the tumor microenvironment where the FAP-alpha enzyme is present, FAP cleaves the blocking group, and allows the radioligand to bind to the tumor’s receptors.
“We are driving innovation in radiopharmaceuticals by overcoming limitations in supply chain, as well as advancing ligand technology,” Dr. McCann says.
“CanSEEK, for example, has the potential to expand the therapeutic window of radiopharmaceuticals and allow for use in earlier lines of disease. In addition, we are working with providers of emerging alpha, beta and auger emitting isotopes to increase potency, and explore the impact of combining radioligands with other treatments.”
• • • • •
To connect with POINT Biopharma Global or any of the other companies featured on BioTuesdays, send us an email at [email protected].






