Altamira Therapeutics (NASDAQ:CYTO) is using its recent acquisition of closely-held Trasir Therapeutics as a steppingstone for a strategic repositioning to focus on development of RNA therapeutics, while in the medium term aiming to spin off or divest its existing assets in neurotology, rhinology and allergology.
“We understand investors prefer pure play company strategies and, in our case, we aim to be a leading company for effective delivery of RNA payloads to tissues beyond the liver,” chairman and CEO, Thomas Meyer, Ph.D., says in an interview with BioTuesdays.
Last week, Altamira changed its name from Auris Medical Holding to better reflect its strategic repositioning.
“Right now, we are an eclectic collection of programs and products, ranging from an early-stage commercial OTC consumer health product, a nasal spray in Phase 2 development and legacy projects about the inner ear.” he adds.
“Even though we continue to believe that our existing business holds great promise, the acquisition of Trasir is the result of a strategy review process initiated and announced in September 2020 to change the strategic direction of our company, Dr. Meyer points out.
“We aim to unlock and create significant shareholder value over the next 12-to-18 months through a strategic repositioning and transformation of Altamira,” he contends. “While we are not under any pressure to dispose of non-core assets, the intent is to sharpen our focus.”
Trasir is the brainchild of Samuel Wickline, M.D., a pioneer in extrahepatic delivery of nucleic acids. Dr. Wickline did the majority of his Trasir work while at Washington University in St. Louis and a few years ago, he moved to University of Southern Florida at Tampa. Dr. Wickline, who is now CSO of Altamira, has had more than 15 years of NIH-funded research to deliver RNA payloads inside cells that are not in the liver.
Dr. Meyer explains that Altamira is developing a versatile peptide-based platform called OligoPhore or SemaPhore, for the safe and effective delivery of nucleic acid payloads, such as small interfering ribonucleic acid (siRNA) and messenger ribonucleic acid (mRNA) into target cells, using systemic or local administration.
siRNA is one type of oligonucleotide, which can be used therapeutically to silence disease-related genes. mRNA can be used therapeutically to translate genetic code from DNA into proteins, which then can be used to replace abnormal or deficient proteins or make proteins to fight or prevent disease.
Dr. Meyer points out that the global market for RNA therapeutics has been growing fast, exceeding $1-billion in 2020. However, appropriate delivery technologies have remained a key rate-limiting step for unlocking their potential.
For example, he says viral-based delivery vectors suffer from lack of transduction efficiency and target specificity, and lipid nanoparticles and currently available ligand conjugates preferentially target the liver, which is fine if that’s the target, but tend to have a suboptimal therapeutic index delivering RNA to other tissues.
On the other hand, he points out that Trasir’s ground-breaking OligoPhore technology has the potential to effectively deliver RNA payloads to tissues beyond the liver, which is inadequately addressed with current delivery approaches. “We look forward to applying OligoPhore for the development of truly innovative RNA therapeutics and advancing them to clinical proof of concept,” he adds.
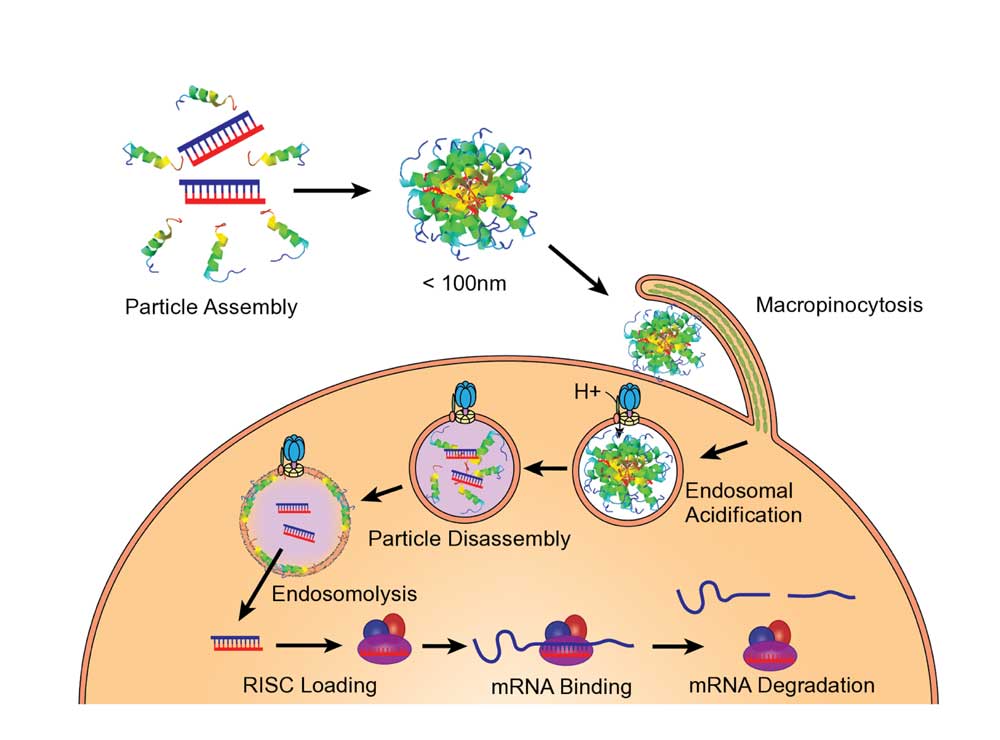
According to Dr. Meyer, OligoPhore’s mechanism of action enables a stable peptide-siRNA polyplex to be taken into the cell through a process called macropinocytosis. Once inside the cell, the endosome is acidified and as the pH drops, the polyplex is disassembled, and the peptide helps the RNA sequence to get outside the endosome, he adds.
OligoPhore enables delivery to target tissues outside the liver, creating the potential for developing RNA-based therapies for a range of indications with substantial unmet need.
In various mouse models of disease, OligoPhore and its mRNA SemaPhore have been shown to protect either the siRNA or mRNA payloads from degradation in the circulation. Proof-of-concept for efficient delivery and target knockdown of undesired genes has been demonstrated for various gene targets, enabling a preclinical development pathway for several oncology indications, rare diseases and various inflammatory conditions.
Based on work of Dr. Wickline and collaborators, the company has selected mutant KRAS-driven colorectal cancer as the first therapeutic indication for its OligoPhore oligonucleotide delivery platform. The company intends to develop the treatment under project code AM-401, targeting an IND submission before the end of 2022.
Colorectal cancer is the fourth most diagnosed cancer and the second leading cause of cancer death in the U.S. Mutations in KRAS alone account for some one million deaths per year worldwide. Approximately 40% to 50% of patients with colorectal cancer harbor mutations of the KRAS gene, which are known to contribute to the development and metastasis of colorectal cancer.
Although the role of KRAS mutations in cancer has been known for decades, they have remained a challenging target for therapeutic treatments. Only recently two small molecule inhibitors of single KRAS mutations were approved by FDA for the treatment of non-small cell lung cancer. Besides colorectal cancer, KRAS mutations are frequently observed in pancreatic cancer and lung cancer.
In parallel with AM-401, Dr. Meyer says Altamira will explore further potential applications of the OligoPhore platform for delivery of siRNA, mRNA, and gene editing constructs, and seek to leverage the platform’s potential through strategic partnering.
“We are not interested in becoming purely a platform company but intend to develop our own program of RNA therapeutics as well,” he adds. The initial focus is on existing siRNA data, while advancing research on mRNA and other payloads, and upscaling manufacturing and developing a safety package for an IND.
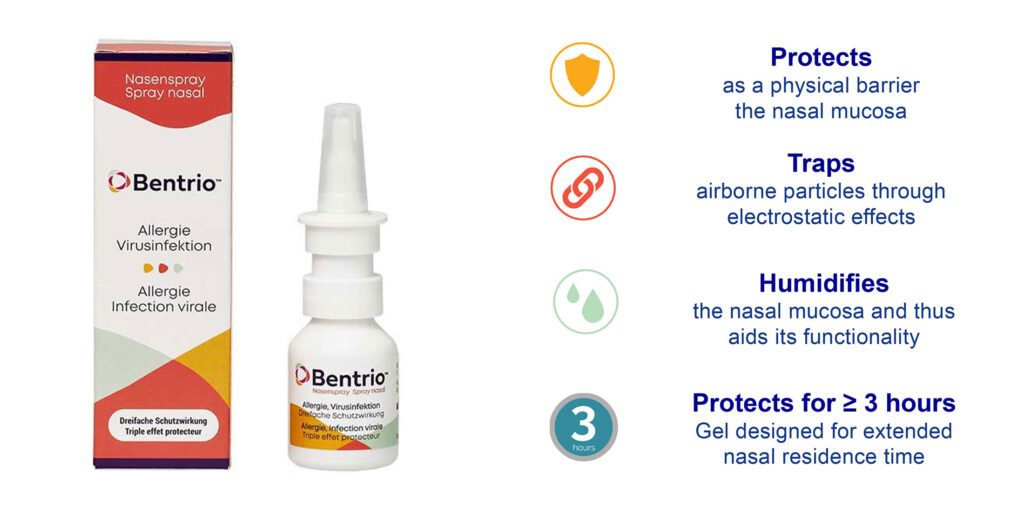
In its historic respiratory business, the company’s OTC nasal spray, Bentrio, is indicated for protection against airborne viruses and allergens. The company is currently launching CE-marked Bentrio in Germany and is readying a 510(k) submission in the U.S. for the treatment of allergy. “These are multibillion-dollar markets,” Dr. Meyer contends.
Bentrio forms a protective thin film gel layer on the nasal mucosa to prevent the contact of viruses or allergens with cells. In addition, the composition of Bentrio serves to bind these particles through electrostatic effects and help with their discharge and to humidify the nasal mucosa. “Together, this is designed to reduce the risk of upper respiratory tract viral infections and promote alleviation of allergic symptoms for more than three hours,” Dr. Meyer points out.
In a clinical pollen chamber open-label cross-over study with 36 patients with allergic rhinitis to grass pollen, a single dose of Bentrio demonstrated a significant reduction in the main symptoms of allergic rhinitis, with the protective effect setting in rapidly and lasting for four hours.
In September 2020, Altamira demonstrated that a key component of its Bentrio nasal spray, AM-301, trapped up to 99% of the Sars-CoV-2 virus, which causes COVID-19, when brought into contact with a viral suspension. In December, the company also obtained direct evidence that AM-301 has the potential to significantly mitigate the risk of infection of nasal mucosal cells.
“The positive market reaction to the announcement of our great SARS-CoV-2 data with AM-301 in early December 2020 showed that investors do appreciate new projects,” Dr. Meyer says. “At the same time, it was not enough for reaching the critical mass we needed.”
Dr. Meyer says Altamira soon plans to start a clinical trial in India to advance the Sars-CoV-2 program, with a readout planned by the end of the year.
“Bentrio has a great market potential addressing frequent viral infections, allergies and air pollution, where more than 90% of the world’s population is exposed to unhealthy air, including China, which has 1.8 million premature deaths from air pollution.”
In its neurotology program, Altamira is developing a reformulated betahistine nasal spray, AM-125, for the treatment of vertigo. The company is completing enrolment in a Phase 2 proof-of-concept TRAVERS trial, with a readout by the end of 2021.
An interim analysis based on Part A of the Phase 2 trial demonstrated a positive dose-response relationship with active-treated patients performing up to 1.9 times to 2.4 times better in the two key balance tests. The company hopes to begin a Phase 3 trial in early 2022.
Oral betahistine tablets are no longer marketed in the U.S. because of efficacy issues but they remain the standard of care in the rest of the world, generating annual sales of about $450-million. “AM-125 addresses betahistine’s weak point: poor oral bioavailability of about 1%.”
In addition, Altamira has two other legacy projects under development – Sonsuvi, for acute inner ear hearing loss, and Keyzilen for acute inner ear tinnitus – that require Phase 3 studies, which could cost around $30-million.
“Because we are prioritizing RNA therapeutics, which is a high potential field, we are seeking to partner these legacy programs,” he says, adding that these are important markets because there are no FDA-approved treatments for acute inner ear hearing loss and acute inner ear tinnitus.
“Our key message is that we are a diversified company now that will become a pure play RNA-focused company over time, unlocking shareholder value in the process,” Dr. Meyer says. “And as we are going through this transformation, we have adopted a new corporate name to reinforce this new direction.”
• • • • •
To connect with Altamira or any of the other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.