Peptilogics is working to change the treatment paradigm of periprosthetic joint infection (PJI), a serious condition that can result from joint replacement surgery. The company is preparing for a registrational clinical trial to begin in early 2024 with its lead asset, PLG0206, to address PJI, an unmet medical need.
“Total joint replacement is the most common major operative procedure in the world, with outcomes that are clinically and economically catastrophic,” Jonathan Steckbeck, Ph.D., founder and CEO of closely-held Peptilogics, says in an interview with BioTuesdays.
“The current standard of care has up to a 60% failure rate at four years and a 25% five-year mortality rate, which is higher than many cancers,” he adds.
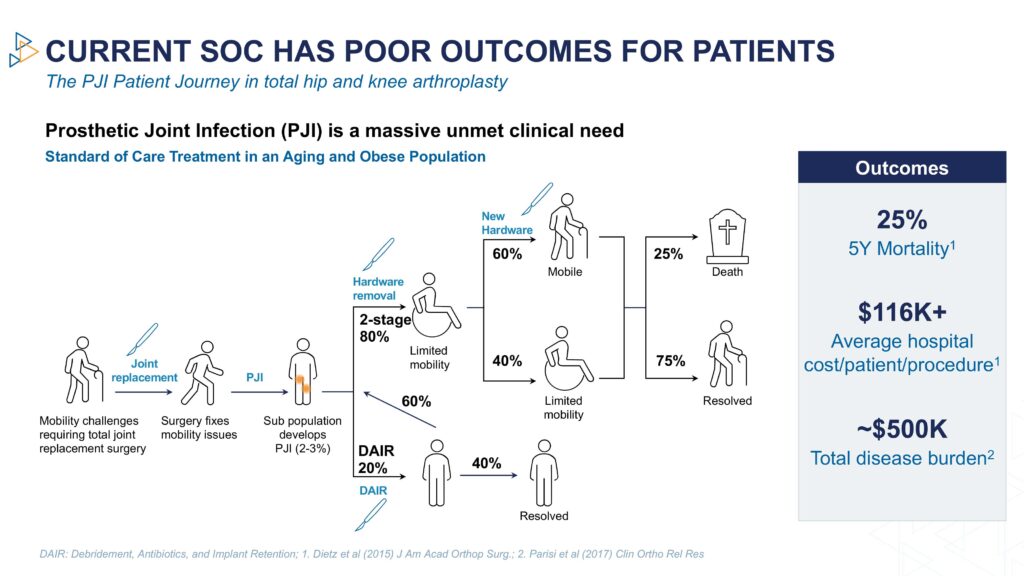
Dr. Steckbeck says that at any time following joint replacement, patients can develop an infection in the new joint, a serious and life-threatening condition. The current standard of care is systemic antibiotics, which have a high failure rate because of the growth of biofilms that block the benefit of traditional antibiotics.
As a result, patients often require additional surgeries, with a 20% increase in mortality for each additional surgery, exacerbated by the lack of mobility from removal, and the eventual replacement of the artificial joint.
“Even with these aggressive interventions, the reinfection rate is about 23%. Physicians and patients are seeking a better option than the current standard of care for PJI,” he contends.
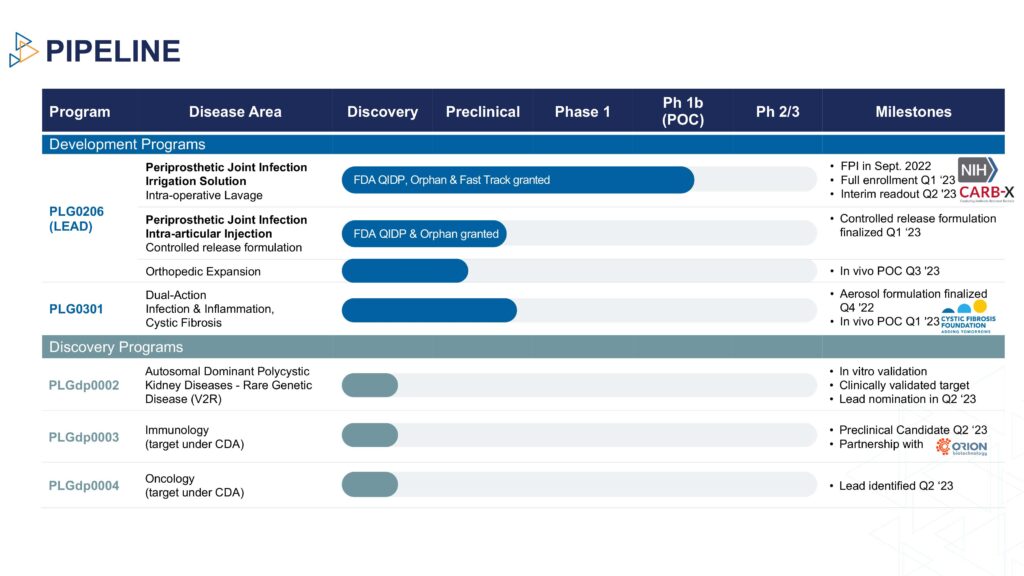
Dr. Steckbeck explains that PLG0206 is a broad spectrum antibacterial, with potent anti-biofilm activity that has received orphan drug, qualified infectious disease product, and fast track designations from the FDA to treat PJI.
“PLG0206 is designed to treat orthopedic infections because its mechanism of action is different than antibiotics.”
Unlike existing antibiotics, Dr. Steckbeck says PLG0206, was engineered to directly address the biofilm and the persister pathogens that make PJI so difficult to treat. It also can be administered locally into the infected site, while maintaining a very low resistance profile and a lower level of inflammation than other molecules in its class.
In early in vitro studies, PLG0206 eliminated biofilm bacteria below detectable levels in five-to-15 minutes. In a preclinical model, 60% of rabbits were cured after being treated with PLG0206 plus standard of care, consisting of surgical saline washout followed with systemic cefazolin, an antibiotic given before certain types of surgery to prevent infections. “This is the first time animal survival was observed,” Dr. Steckbeck says.
In addition, PLG0206’s efficacy was studied in 17 prosthetics surgically removed from PJI patients undergoing knee revision surgery. “94% of the devices were functionally sterilized.”
Last month, Peptilogics reported interim data from a Phase 1b open label study of patients undergoing debridement, antibiotics, and implant retention for treatment of PJI occurring after total knee arthroplasty.
In the low dose cohort with patients observed at three months post-treatment, Peptilogics didn’t observe any reinfections in six of the seven patients participating in the clinical trial, with one patient missing their 90-day follow-up. As a benchmark, studies suggest that reinfection rates for this population can be as high as 44%.
The open-label study enrolled two cohorts of seven patients each, with single-dose escalating treatments of PLG0206 of 3 mg/mL or 10 mg/mL, as an irrigation, compared with historical controls with standard-of-care treatment. An earlier Phase 1a study with PLG0206 delivered intravenously observed no serious adverse events and none of the challenges that have previously limited this class of molecules.
Peptilogics also is developing a controlled-release formulation of PLG0206 as an intra-articular injection, with a Phase 1b trial set to begin in 2024.
Each year, approximately 1.5 million total joint replacements are performed in the U.S., a number Dr. Steckbeck figures is expected to increase to three million by 2030 because of an aging population. Many of the patients have significant comorbidities, which increases the odds of a poor outcome. In 2030, the estimated market for PJI is expected to exceed $5-billion.
The estimated direct cost of a single episode of care for PJI exceeds $100,000 and the average total per patient treatment cost is around $500,000 because of more clinic visits, frequent hospitalizations and longer hospital stays.
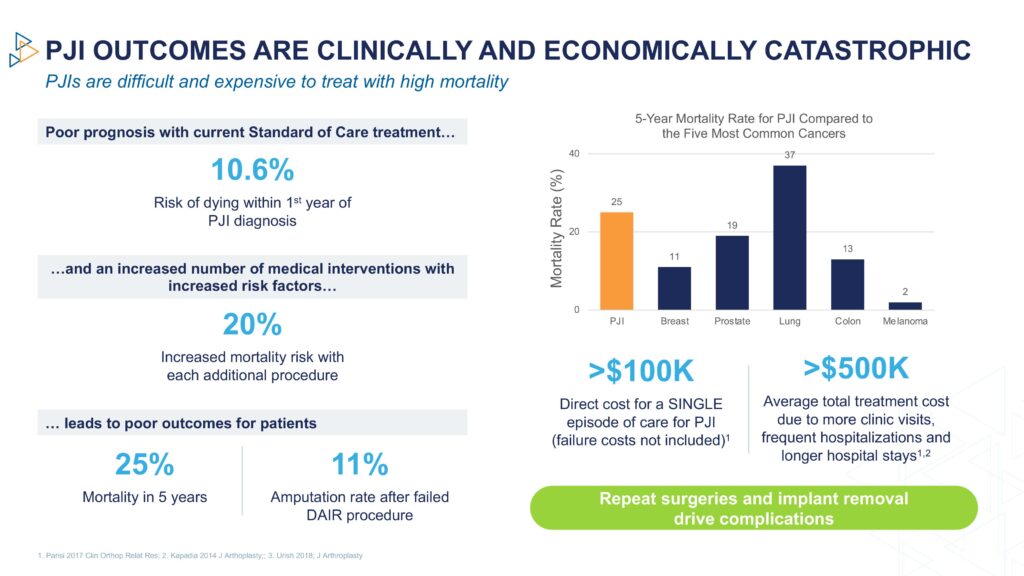
By improving PJI treatment, PLG0206 has the potential to positively impact patients through fewer surgeries, reduced hospitalization, leaving the original hardware in place, and improved mobility, Dr. Steckbeck points out. Surgeons would benefit from lower recurrence rates and increased patient satisfaction, as well as add-on reimbursement. Hospitals and insurance payers stand to benefit from significant downstream savings, he adds.
In addition to the PLG0206 asset, Peptilogics is building a peptide discovery engine, known as Nautilus. “The platform leverages generative machine learning and artificial intelligence to generate high quality peptide candidates at a speed and scale that is unachievable using conventional methods,” Dr. Steckbeck says.
“Early work with partners has shown tremendous results that validate that the engine is better and faster at designing peptides than traditional approaches for historically challenging criteria like specificity, solubility and stability.”
• • • • •
To connect with Peptilogics or any of the other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.