Indaptus Therapeutics (NASDAQ:INDP) is conducting a first-in-human, dose escalation Phase 1 study of its lead compound, Decoy20, in patients with advanced metastatic solid tumors where currently approved therapies have failed.
“Our approach is based on the observation that tumor regression can occur in the presence of a bacterial infection,” Jeffrey Meckler, CEO of Indaptus, says in an interview with BioTuesdays.
This has been known for about 1,000 years and more recently, it has been shown that bacteria don’t need to be alive or cause an actual infection to produce tumor regression.
“Bacteria’s natural ability to activate both innate and adaptive immune cells and associated antitumor immune responses form the basis of our Decoy platform,” he says, adding that the technology has applications across oncology, infectious diseases, and immunology.
The challenge has been that this approach works best when bacteria are administered intravenously, but bacteria found in nature, even if killed, also produce excessive toxicity when given by IV.
Mr. Meckler explains that Indaptus’ technology starts with engineered bacteria and then reduces the level of lipopolysaccharide (LPS) activity by about 90%. By leaving a small amount of LPS activity associated with the bacteria, both innate and adaptive systemic immune responses can be activated with much safer IV administration. The bacteria are then processed so to ensure 100% of the bacteria is killed and stabilized to produce the Decoy product.
The Indaptus Approach
Indaptus Therapeutics was formed in 2021 through the merger of Intec Pharma and Decoy Biosystems. The company’s technology is currently protected by five issued patents in the U.S, as well as 29 issued or granted patents outside the U.S., including Europe, covering compositions, methods of making and methods of treatment that are based on technology originally developed at Decoy Biosystems by founder and CSO of Indaptus, Michael Newman, Ph.D.
In standard preclinical models, “Decoy Therapeutics has produced significant single agent activity against metastatic pancreatic and colorectal carcinomas; single agent eradication of established, antigen-expressing breast carcinoma; and combination-mediated eradication of established hepatocellular carcinomas and non-Hodgkin’s lymphomas, including both mouse and human tumors,” Mr. Meckler points out.
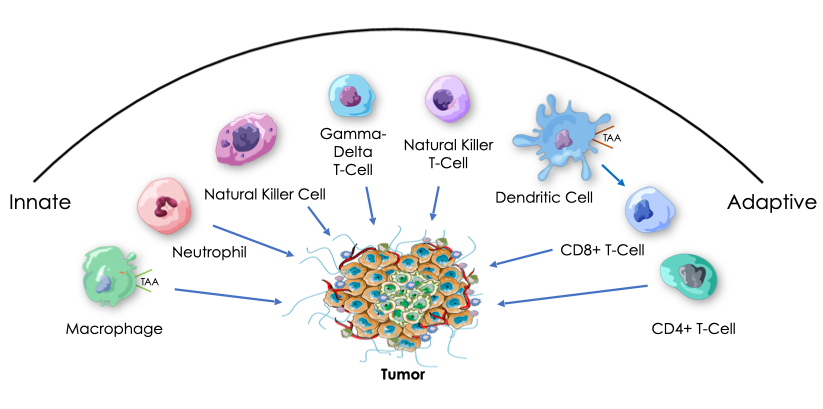
Tumor regression is seen with up to 100% of mice and mice that have been cured by Decoy combination therapy reject fresh tumor cell challenge via both innate and adaptive immune responses, an extremely important phenomenon called immunological memory.
Tumor eradication has been observed with Decoy products in combination with anti-PD-1 checkpoint therapy, low-dose chemotherapy or an approved targeted antibody therapies, he adds.
Mr. Meckler suggests that current cancer immunotherapies only address a limited part of the immune system, leading to low cure rates in advanced cancers.
In preclinical models, tumor eradicating Decoy combinations have been shown to activate both innate and adaptive immune pathways in tumors after just one week of treatment, including just one Decoy treatment. Tumor eradication has also been shown to involve natural killer, CD4+ T and CD8+ T immune cells. Preclinical data also support a mechanism of action involving additional immune cells, including macrophages and dendritic or antigen presenting cells.
“Effectively, we are tricking the body into thinking there is something that needs activation of both sides of the immune system,” Mr. Meckler suggests.
The challenge, however, is that intravenous administration of Gram-negative bacterial is toxic, reflecting very high amounts of LPS, which constitute some 75% of the Gram-negative outer cell membrane. “Our approach is to significantly reduce LPS but leave enough to activate innate and adaptive immune responses,” he adds.
Indapatus’ IND-enabling toxicology studies did not produce evidence of cytokine release syndromes, which are relatively common with several different types of immuno-oncology therapies.
Decoy drug candidates also have shown significant single agent activity in preclinical models of hepatitis B virus and HIV.
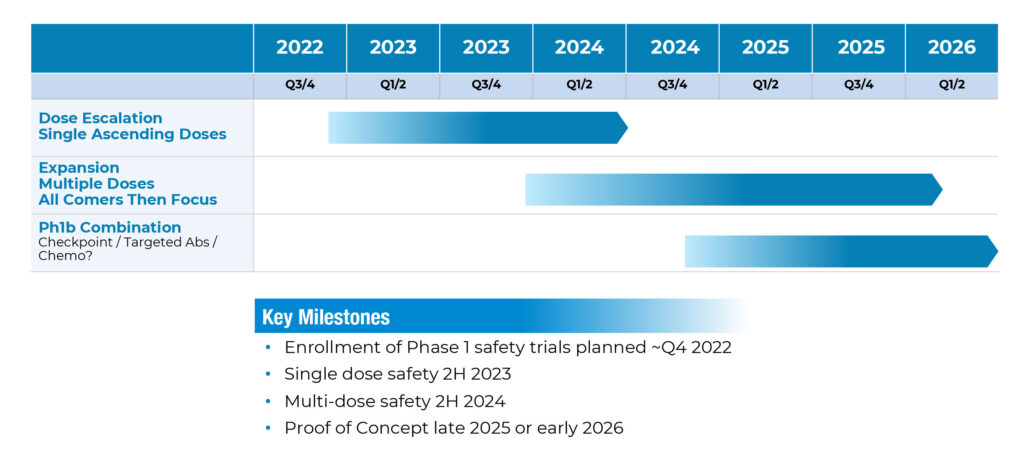
The company’s Phase 1 study in patients with advanced or metastatic solid tumors is designed to evaluate the safety, tolerability, and preliminary efficacy of single doses of Decoy20 in three subject dose-escalation cohorts, followed by weekly dosing with new patients at the recommended Phase 2 dose , with data readouts in the second half of 2023 and second half of 2024, respectively.
Mr. Meckler says Part 3 of the study would focus on specific solid tumor indications with a combination therapy approach. “We hope to establish proof of concept in late 2025 or early 2026.”
The primary endpoint of the study is incidence, relatedness and severity of adverse events and treatment-emergent adverse events and determining the single and weekly dose levels that can be given with an acceptable side-effect profile.
Secondary endpoints include Decoy20 pharmacokinetic parameters; and objective response rate and duration of response in subjects with measurable disease.
“The Phase 1 study is an important step in our efforts to understand the potential of Decoy20 to treat a wide range of solid tumors, including hepatocellular, colorectal and pancreatic carcinomas,” Mr. Meckler says. “The need is great since the five-year survival rate for patients with the most deadly metastatic tumors is 3% to 17%.”
Indaptus Pipeline
• • • • •
To connect with Indaptus Therapeutics or any of the other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.