Avidity Biosciences (NASDAQ:RNA) is developing a novel approach for the delivery of RNA therapeutics with a new class, known as antibody oligonucleotide conjugates (AOCs), with an initial focus on skeletal muscle diseases.
“Our AOCs are designed to combine the specificity of monoclonal antibodies with the precision of oligonucleotide therapies to target the root cause of diseases previously untreatable with RNA therapeutics,” Sarah Boyce, president and CEO of Avidity, says in an interview with BioTuesdays.
“The flexibility of our AOC platform allows us to deploy various types of oligonucleotides, including double-stranded small interfering RNAs (siRNAs) and single-stranded splice skipping oligonucleotides with phosphorodiamidate morpholino oligomers (PMO) chemistry, each of which modify RNA function in different ways. This allows us to use the oligonucleotides that are tailored to modulate a specific disease process.”
Ms. Boyce explains that each component of an AOC has been engineered for optimal delivery to muscle tissue, with well-established safety. Approved siRNA drugs have demonstrated no known liver or renal toxicity as well as no thrombocytopenia, or a condition of low platelet count that can impede wound healing. Approved PMO drugs have demonstrated safety and activity, she adds.
The company has advanced three AOCs into clinical development, including two siRNAs and one PMO.

Avidity’s muscle tissue programs include:
- AOC 1001 for the treatment of people with myotonic dystrophy type 1 (DM1) is in Phase 1/2 clinical development with the ongoing MARINA and MARINA-OLE trials.
- AOC 1020 to treat people living with facioscapulohumeral muscular dystrophy (FSHD) is in Phase 1/2 clinical development with the FORTITUDE trial.
- AOC 1044 for people with Duchenne muscular dystrophy (DMD) mutations amenable to exon 44 gene skipping. AOC 1044 is in Phase 1/2 clinical development with the EXPLORE44 trial and is the first of several AOCs the company is developing for DMD.
Avidity is also broadening the reach of AOCs beyond skeletal muscle through both internal discovery efforts and key partnerships in areas such as cardiac tissue, immunology and other cell and tissue types.
There are no approved therapies to treat DM1, which affects more than 40,000 people in the U.S. and a similar number in Europe. DM1 is a progressive disease, with varying symptoms from patient to patient but primarily affecting skeletal, cardiac and smooth muscle.
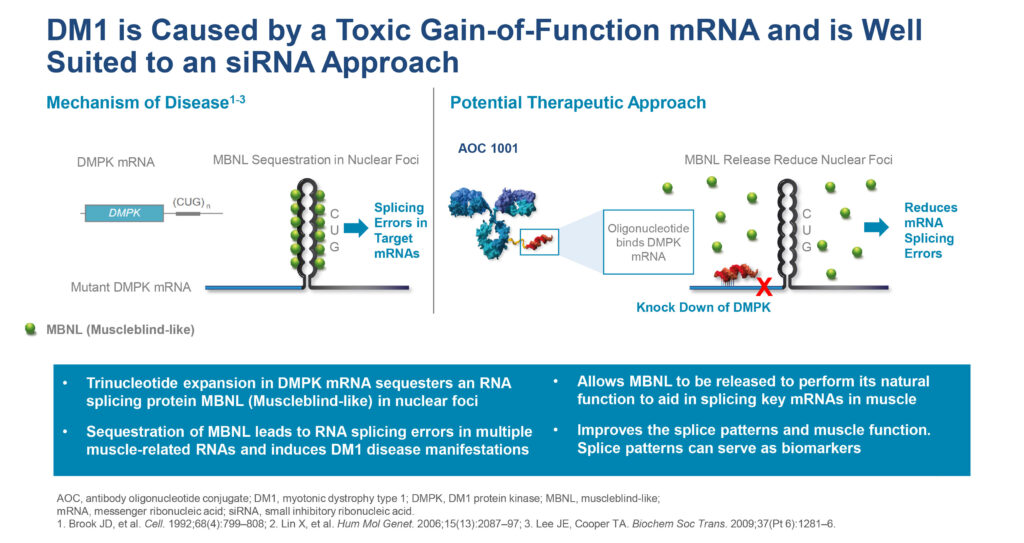
The disease, which is caused by mutations in the DMPK gene, increases in severity from generation to generation, with a significant impact on quality of life and shortened life expectancy.
According to Ms. Boyce, Avidity’s AOC 1001 is designed to address the root cause of DM1 by reducing levels of disease-related mRNA in skeletal, cardiac and smooth muscle.
In preclinical studies, AOC 1001 successfully delivered siRNAs to muscle cells, resulting in durable, dose-dependent reductions of DMPK RNA across a broad range of muscles, she adds.
In addition, IND-enabling 13-week toxicity studies in non-human primates demonstrated no dose limiting toxicity at the highest dose tested as well as no observed platelet or renal toxicity.
AOC 1001 is currently in Phase 1/2 development with the ongoing MARINA clinical trial in adults with DM1. Current participants in the MARINA study are eligible to enroll in the MARINA-OLE open-label extension study. The FDA and European Medicines Agency have granted orphan drug designation for AOC 1001 and the FDA has granted AOC 1001 fast track designation.
In September 2022, the FDA placed a partial clinical hold on new participant enrollment in the MARINA study in response to a serious adverse event reported in a single participant in the 4 mg cohort.
Ms. Boyce explains that all current participants, whether they are on AOC 1001 or placebo, may roll over to the 24-month MARINA-OLE study, where they will receive AOC 1001.
“We are conducting a thorough analysis and working closely with the FDA and trial investigator to resolve the recent partial clinical hold on new participant enrollment as soon as possible,” she adds.
Close to 40 participants are currently enrolled in the MARINA study and Ms. Boyce says Avidity is on track for a preliminary assessment of the 1 mg and 2 mg cohorts before the end of 2022. Top-line data is scheduled for release in 2023.
“At the mid-point assessment, we’ll take a look at the data to determine the best path forward to a pivotal trial,” she suggests.
“We share the sense of urgency with the DM1 community for effective therapies and we remain confident that AOC 1001 has the potential to address important unmet needs in people living with DM1.”
In September 2022, the FDA cleared the company’s IND applications of AOC 1020 for FSHD, its second muscle-targeting siRNA AOC, and for AOC 1044 to treat DMD mutations amenable to exon 44 gene skipping.
The FORTITUDE trial is a randomized, placebo-controlled, double-blind, Phase 1/2 clinical trial designed to evaluate AOC 1020 in 68 adult participants with FSHD, for which there are no approved therapies. AOC 1020 shares the same antibody as AOC 1001 conjugated with a therapeutic siRNA that is designed to reduce the inappropriate expression of the disease-driving gene DUX4.
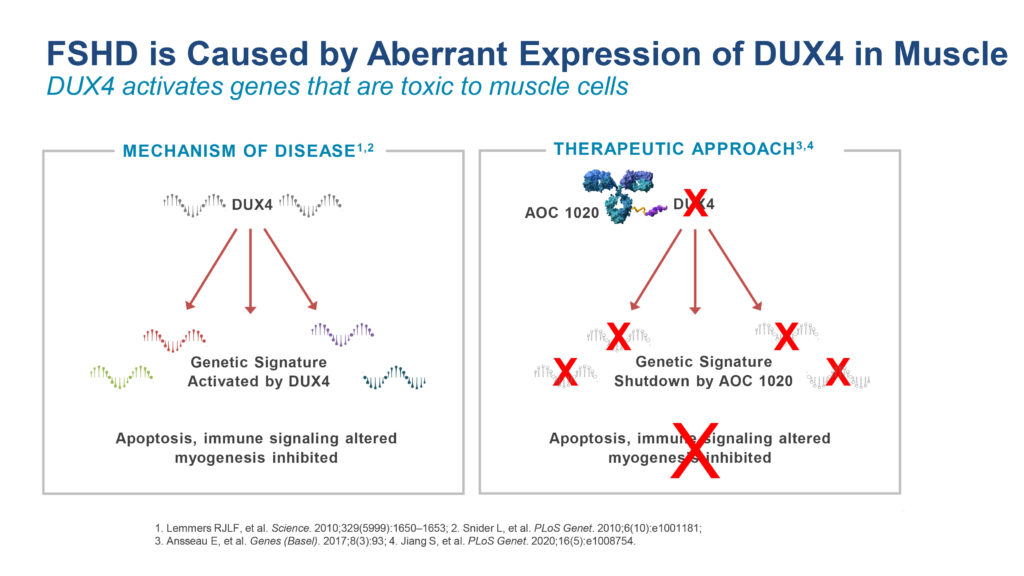
FSHD, an inherited condition, affects approximately 16,000-to-38,000 people in the United States alone. It is an autosomal dominant disease caused by the abnormal expression of the gene DUX4 that leads to skeletal muscle wasting and progressive loss of muscle function, with symptoms often beginning in adolescence and early adulthood.
FORTITUDE will evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of AOC 1020 administered intravenously, with the primary objective being the safety and tolerability of AOC 1020 in FSHD patients. Activity of AOC 1020 will be assessed using key molecular biomarkers, including MRI measures of muscle volume and composition.
Ms. Boyce points out that while the Phase 1/2 trial is not statistically powered to assess functional benefit, it will explore the clinical activity of AOC 1020, including measures of mobility and muscle strength as well as patient reported outcomes and quality of life measures. Participants will have the option to enroll in an open-label extension study at the end of the treatment period in the FORTITUDE study, which will have a preliminary assessment in the first half of 2024.
Avidity’s first PMO to enter the clinic is AOC 1044 for the treatment of DMD, a rare, genetic condition that is characterized by progressive muscle damage and weakness. Those living with the condition often require special aid and assistance throughout their lives and have significantly shortened life expectancy.
AOC 1044 is designed for people living with DMD amenable to exon 44 skipping. AOC 1044 is the first of multiple AOCs the company is developing for DMD, followed by AOCs designed to target exon 45 and exon 51.
Currently, there are no approved therapies to treat the underlying mechanism of disease for people living with DMD mutations amenable to exon 44 skipping.
DMD is an irreversible, progressive disease caused by a genetic mutation that prevents the body from producing a protein called dystrophin, which protects muscle cells from injury during contraction. The lack of functional dystrophin leads to muscle cell death and progressive loss of muscle function.
AOC 1044 is designed to deliver PMOs to skeletal muscle and heart tissue to specifically skip exon 44 of DMD to enable the production of a slightly shortened but functional version of the dystrophin protein.
Avidity’s EXPLORE44 trial is a randomized, placebo-controlled, double-blind, Phase 1/2 clinical trial to evaluate AOC 1044 in healthy volunteers and participants with DMD mutations amenable to exon 44 skipping (DMD44). EXPLORE44 will evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamic effects of single and multiple ascending doses of AOC 1044 administered intravenously.
EXPLORE44 is expected to enroll approximately 40 healthy volunteers and 24 participants with DMD44, ages seven to 27. The EXPLORE44 trial will assess exon skipping and dystrophin protein levels in participants with DMD44. Participants with DMD44 also will have the option to enroll into an extension study. The company expects to report results from healthy volunteers in the second half of 2023.
“Our mission is to profoundly improve people’s lives and we have now advanced three programs – DM1, FSHD and DMD44 – into clinical development in a 14-month period,” Ms. Boyce says.
• • • • •
To connect with Avidity Biosciences or any of the other companies featured on BioTuesdays, send us an email at [email protected].