
With last month’s FDA clearance of the SavvyWire transcatheter aortic valve replacement (TAVR) guidewire, OpSens (TSX:OPS; OTCQX:OPSSF) is bringing a new concept to interventional cardiology and the delivery of new heart valves.
“SavvyWire is a game changer for us as the world’s first and only sensor-guided TAVR solution,” Louis Laflamme, president and CEO of OpSens, says in an interview with BioTuesdays.
The company’s breakthrough culminates a steadily growing cardiovascular business based on a platform of using fiber optics to measure pressure and temperature. SavvyWire follows an earlier guidewire product, known as OptoWire, which is used for physiological measurements in delivering stents, for example.
“SavvyWire provides the first three-in-one solution for stable aortic valve delivery and positioning, continuous accurate hemodynamic measurement during the procedure, and reliable left ventricular pacing without the need for adjunct devices or venous access,” he adds.
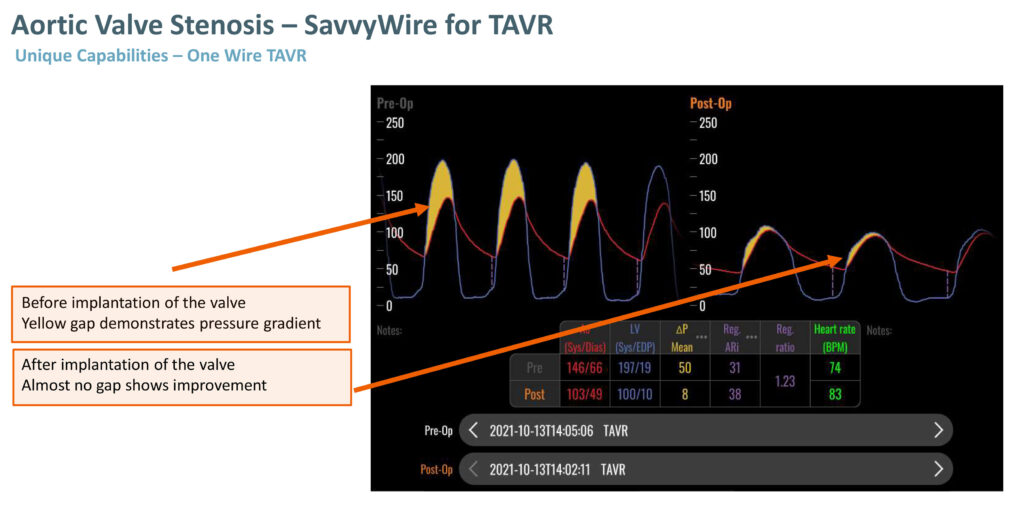
While competitors such as Boston Scientific and Medtronic have guidewires to deliver a heart valve, they require additional devices to measure hemodynamic information and perform ventricle pacing, which is key to the TAVR procedure.
The TAVR procedure, one of the fastest growing med-tech end markets, was initially only indicated for inoperable patients with severe symptomatic aortic stenosis, and later for patients at high surgical risk.
Aortic valve stenosis occurs when the heart’s aortic valve narrows, preventing the valve from opening fully, which reduces or blocks blood flow from the heart into the aorta and the rest of the body. Previously, the condition required open heart surgery.
Mr. Laflamme says SavvyWire introduces an entirely new category of innovation to the structural heart device market segment. “Our new guidewire allows for a single wire to measure hemodynamic information, stimulate the heart and deliver the valve, potentially reducing complications, saving time and money.”
The TAVR procedure is rapidly evolving toward a minimally-invasive approach that allows patients to leave the hospital earlier, sometimes the same day after their treatment.
The global TAVR market is currently estimated at over 200,000 procedures a year and is expected to reach 400,000 in 2027, which Mr. Laflamme says should more than double the value of the market to almost $10-billion.
Soon after the FDA clearance of SavvyWire last month, the guidewire won high marks from Dr. Philippe Genereux of Morristown Medical Center in New Jersey after he successfully treated 10 consecutive TAVR patients with a variety of cardiac conditions in the first use of SavvyWire in the U.S.
“There is no doubt the SavvyWire allowed us to optimize our efficiency and workflow, while enhancing accuracy and patient safety,” Dr. Genereux said in a statement. “I am excited to collaborate with OpSens to bring this disruptive technology to patients.”
Mr. Laflamme says OpSens is conducting a controlled release of SavvyWire to key opinion leaders and a limited number of hospitals in the U.S. through the end of 2022, with a full launch planned in early 2023. The company expects to seek marketing approval of SavvyWire in Europe in 2023 and Japan in 2024.
OpSens initially cut its teeth on developing, manufacturing, and installing innovative fiber optic sensing solutions for medical and critical industrial applications. “The company was founded on a technology using light to measure things like pressure and temperature,” Mr. Laflamme recalls.
And in its early days, OpSens developed several business partnerships with original equipment manufacturers, such as Abiomed and Monteris, giving them rights to exploit its sensors in specific areas.
Abiomed, for example, integrates OpSens technology in its Impella Cardiac Pump under a supply agreement until 2028 while OpSens technology is incorporated in Monteris’ Neuroblate Optic Laser probes for laser ablation with fiber optic temperature sensor.
OpSens’ initial success supplying partners led the company to develop its flagship product, OptoWire, an advanced optical-based pressure guidewire to improve the clinical outcome of patients with coronary artery disease.
OptoWire is a second-generation fiber optic pressure guidewire that measures fractional flow reserve and diastolic pressure ratio, and helps cardiologists determine the type of treatment a patient requires. It also provides the lowest drift in the industry and excellent lesions access.
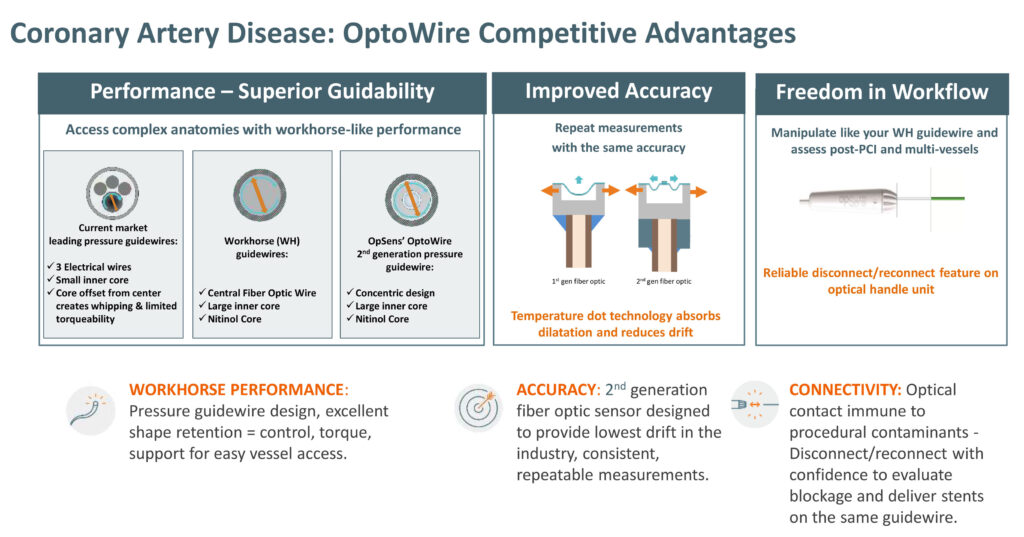
The OptoWire has been used in the diagnosis and treatment of more than 150,000 patients with coronary heart blockages in more than 30 countries and also led to the installation of more than 2,000 OptoMonitor units in clinics, creating recurring revenue and a base for additional product offerings. OptoWire is approved for sale in the United States, European Union, Japan, and Canada.
Fractional flow reserve measurement involves determining the ratio between the maximum achievable blood flow in a diseased coronary artery and the theoretical maximum flow in a normal coronary artery. The global fractional flow reserve market is expected to reach $1-billion by 2025.
In 2009, in a study known as FAME, researchers demonstrated that when fractional flow reserve measurement is used prior to percutaneous coronary intervention, patient outcomes improved.
Percutaneous coronary intervention refers to a family of minimally-invasive procedures used to open clogged coronary arteries, such as installing a stent, angioplasty and bypass surgery.
For example, a cardiologist would measure blood pressure before and after a blockage to obtain a ratio of fractional flow reserve or diastolic pressure to determine the severity of the blockage and treatment type. If a stent is required, the cardiologist would deliver it to the blockage over the OptoWire.
Mr. Laflamme says OptoWire saves time and costs, reduces the number of devices needed, provides greater accuracy with less stenting. The strategy seems to be working. OptoWire revenue was (Canadian) for the fiscal year ended Aug. 31, 2001, or two-thirds of total revenue, up from $5.2-million in 2016.
OpSens has an approximate 3% market share of the $225-million U.S. market size for OptoWire and recently signed three contracts with national group purchasing companies to expand the commercialization of OptoWire in the U.S. by increasing its access to cardiac catheterization labs.
Mr. Laflamme says OpSens has contracts in place covering more than 90% of hospitals in the U.S. through group purchasing agreements.
The company uses distributors to sell OptoWire in Japan, Europe, the Middle East and Africa, has a 15% share of the $20-million market in Canada through direct sales.
“Our strategy in developing SavvyWire was to build a sales team to sell two products to the same doctors that know our OptoWire product,” Mr. Laflamme says. OpSens plans to build a team of 25 sales managers by the end of 2022 in preparation for the launch of SavvyWire in 2023.
“We have the best products in growing markets, including the standard of care in the U.S., and we plan to put more effort into making our products better known in the U.S.,” he adds.
OpSens also has a small but growing industrial segment, which applies innovative fiber optic measurement solutions for industrial applications. These include, for example, fuel monitoring in aeronautics; temperature assessment during manufacturing of semiconductors; validation of electro-explosive devices for the military; infrastructure monitoring in the nuclear industry.
In 2021, the company’s OpSens Solutions unit received a contract from RI Research Instruments GmbH to supply fiber optic absolute and differential pressure sensors for the International Thermonuclear Experimental Reactor (ITER) project.
ITER is the world’s largest nuclear fusion and scientific experiment project with 35 nations collaborating to build and operate a potential source of safe, non-carbon emitting and an almost unlimited nuclear fusion-based potential energy source. ITER is currently under construction in southern France.
“We believe this represents worldwide recognition of OpSens’ fiber optic sensing technology and we are optimistic this will be a tremendous business opportunity for the company going forward,” Mr. Laflamme says.
• • • • •
To connect with OpSens or any of the other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.






