
As a research assistant professor at the Department of Pharmacology at the University of Washington, Dr. Ben Land, Ph.D. is broadly interested in how cannabinoids and opioids mediate pain and reward. But in a collaboration between Dr. Charles Chavkin’s laboratory at the University of Washington and Titan Pharmaceuticals (NASDAQ:TTNP), Dr. Land evaluated Titan’s novel peptide therapeutic that is in development for treating chronic pruritus, a severe and debilitating clinical condition. In this interview with BioTuesdays, Dr. Land discusses chronic pruritus, Titan’s investigational TP-2021 drug implant and his recent preclinical findings.
What is chronic pruritus and how is it treated?
Chronic pruritus is a condition that affects around 23 million-to-44 million people in the U.S. and is defined as severe itching and scratching that persists for more than six weeks. It is a prevalent and debilitating medical condition associated with some kidney, liver, and chronic skin diseases, such as atopic dermatitis. Treatments like antihistamines, corticosteroids, and over-the-counter lotions have limited efficacy for this condition.
What are you targeting with Titan’s TP-2021?
The kappa opioid receptor system has long been an attractive target because these receptors are located on the primary sensors, or nociceptors, of the pain and itch pathways, and kappa opioid agonists have been shown to reduce chronic itching. Until recently, however, the big challenge has been that most kappa opioid agonists also activate the central nervous system, causing unwanted side effects like dysphoria. Titan’s TP-2021 is highly selective for kappa opioid receptors within the peripheral nervous system and does not penetrate the central nervous system.
Are there kappa agonists for pruritus on the market?
The first kappa agonist shown to work for this condition is a drug called nalfurafine that is approved only in Japan and marketed as Remitch for treating uremic pruritus. It is a small molecule kappa agonist that also penetrates the blood-brain-barrier. Therefore, it has dysphoric side effects due to its action on kappa receptors in the central nervous system in addition to its antipruritic effects from its action on peripheral kappa receptors.
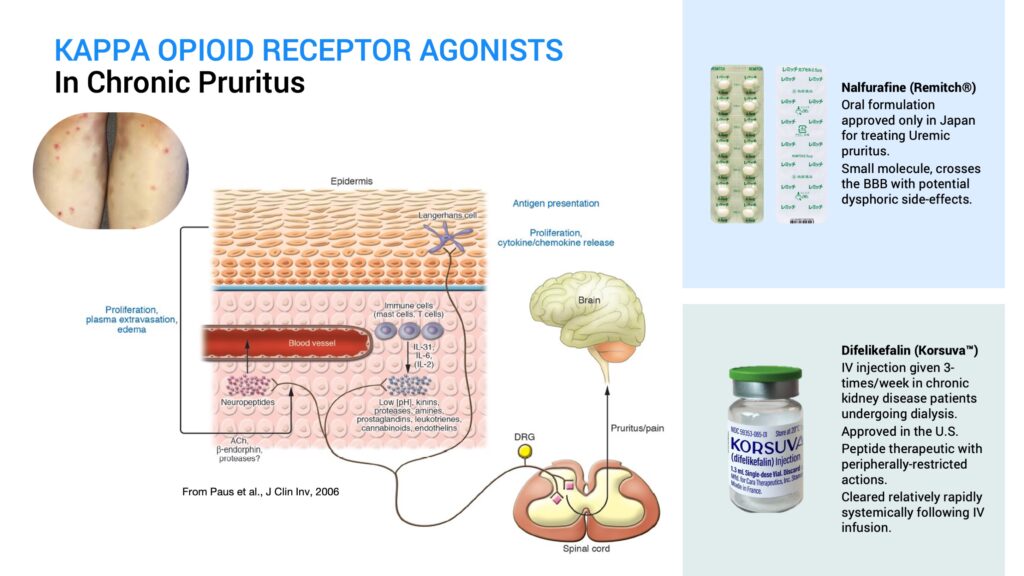
Earlier this year, a novel drug called difelikefalin, which is marketed as Korsuva by CARA Therapeutics, was approved in the U.S. as an IV injection given three times a week in chronic kidney disease patients undergoing dialysis. It is a peptide kappa agonist that is peripherally restricted and doesn’t show central side effects. However, it is systemically cleared relatively rapidly following IV infusion.
So, there is an unmet need for a peripherally-restricted kappa opioid agonist therapeutic for treating pruritus that can be delivered over the long term, even several months at a time from a single treatment, especially for chronic skin conditions, such as atopic dermatitis.
Can you describe TP-2021?
TP-2021 is a novel peripherally-restricted kappa receptor agonist being developed by Titan Pharmaceuticals for treating chronic pruritus. It is a synthetic peptide like difelikefalin and will not cross the blood-brain-barrier and bind to central kappa receptors associated with the dysphoric side-effects. Like difelikefalin, TP-2021 is a highly selective kappa opioid receptor agonist acting at picomolar concentrations, with little to no activity at mu-or-delta opioid receptors even at micromolar levels.
How did you initially assess TP-2021?
To assess the anti-pruritic activity of TP-2021 in comparison to the known drug, difelikefalin, I tested two doses of each drug given subcutaneously by injection in an established mouse model for pruritus. Control animals were injected with a placebo. After 20 minutes, a known scratch-inducing chemical called 5-prime GNTI was given by subcutaneous injection and the animals were video-recorded in 1080p resolution at 60i frames a second. The number of scratching bouts over a 30-minute period was then assessed for each animal in the study.
What did you find?
The results indicated that both TP-2021 and difelikefalin were equally potent in significantly suppressing GNTI-induced scratching at a dose of 0.3 mg per kg, with less efficacy of scratch suppression at a lower dose of 0.1 mg per kg, and with TP-2021 showing slightly better efficacy than difelikefalin at this dose.
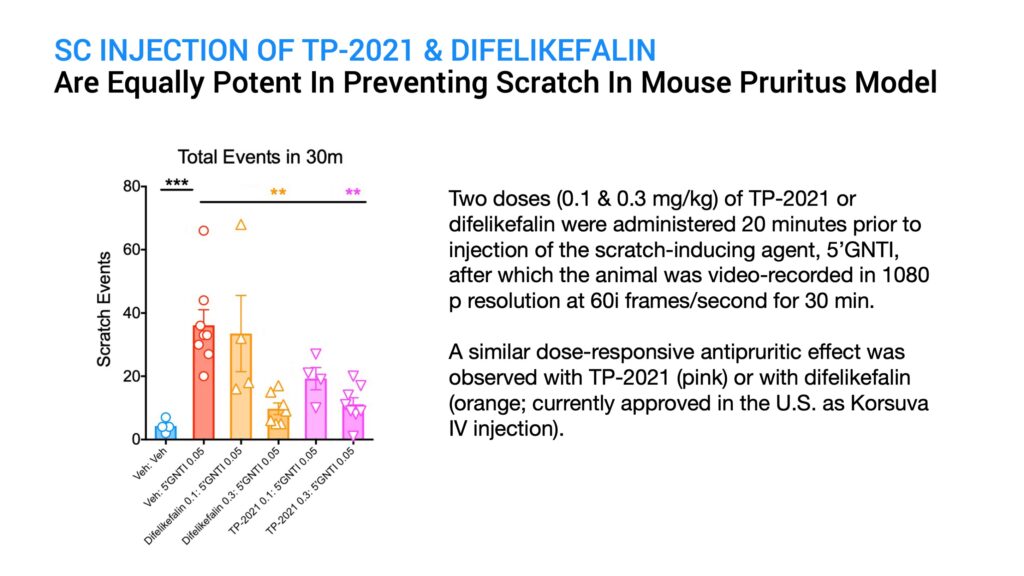
Since the subcutaneous injection of TP-2021 showed equivalent potency with difelikefalin in this animal model, I proceeded to evaluate in this same model the efficacy of Titan’s ProNeura implant formulated for the long-term delivery of TP-2021.
What is ProNeura and how does it work?
ProNeura is Titan’s long-term drug delivery implant technology made up of the safe and inert polymer, ethylene vinyl acetate, blended with a drug of interest that can range from small molecules to peptides and hormones. The mixture is extruded under mild heat and pressure to form the implant, which has the drug embedded in the ethylene vinyl acetate matrix. These implants are placed subdermally and the embedded drug is released into the interstitial fluid through a process of dissolution-controlled diffusion. Following an initial brief peak due to small amounts of drug released from the implant surface, the implants provide non-fluctuating continuous release that is typically sustained for several months after insertion. The duration of sustained release and plasma concentration are controlled by the drug’s potency and the amount of drug contained in the implant. ProNeura implants allow for released drug to bypass first-pass hepatic metabolism and eliminate the peaks and troughs typically observed with oral administration. The first ProNeura-based product, a six-month buprenorphine implant, was approved by regulatory authorities in the U.S., Canada, and the EU for the maintenance treatment of opioid use disorder.
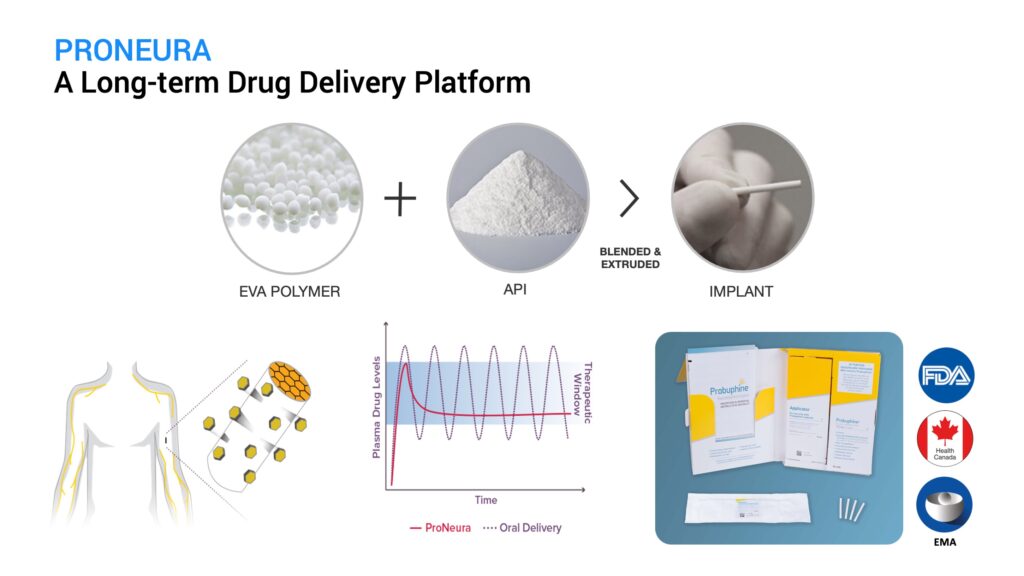
What were the findings that you reported at the Society for Neuroscience meeting on Nov. 8, 2021?
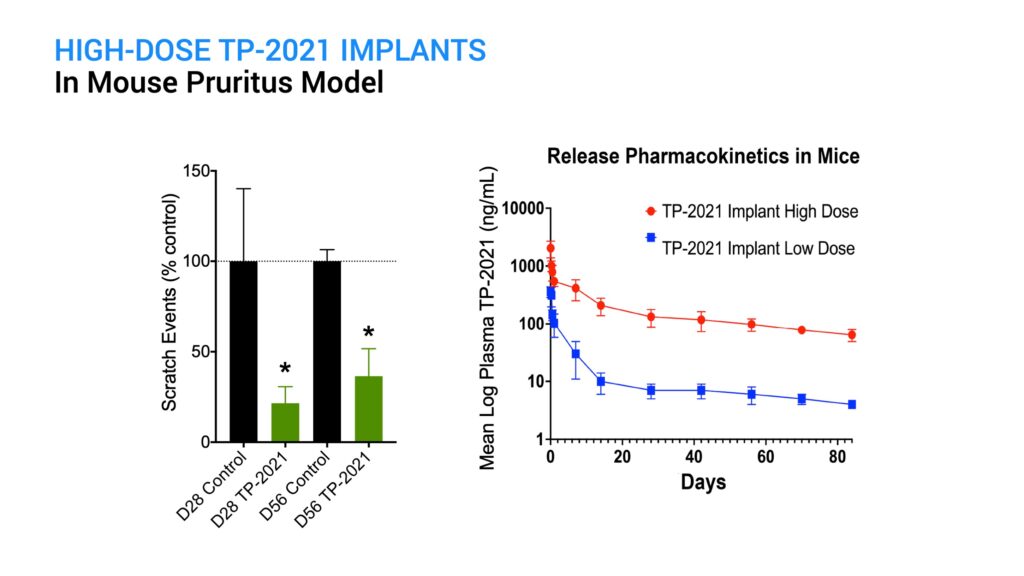
I initially tested ProNeura implants containing a low dose of TP-2021 implanted in mice and saw a reduction in GNTI-induced scratching that was effective for two weeks post-implantation. However, by day 28 the anti-pruritic effect waned and approached control levels. Pharmacokinetic assessment of plasma TP-2021 levels in mice indicated that the loss in activity corresponded to levels falling below about 10 ng per mL, which occurred after day 14.
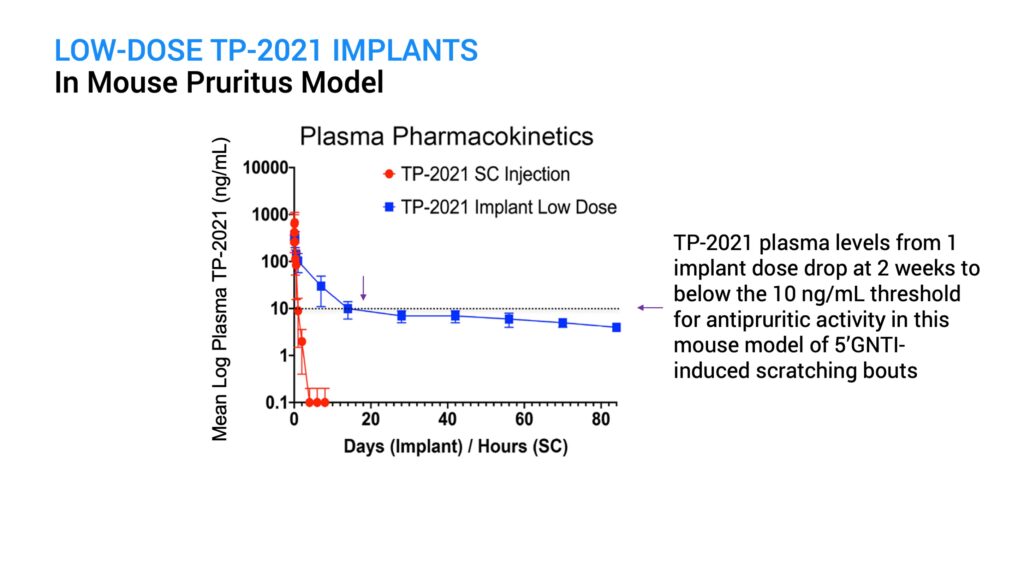
A higher dose TP-2021 implant was next tested in this model to see if it could sustain the anti-pruritic activity for longer than two weeks. At both day 28 and day 56 post-implantation, there was a significant reduction in scratching behavior following GNTI challenge, compared with the control animals. There were no safety issues observed in the implanted animals. Because the high-dose implant provided sustained supra-therapeutic plasma levels of TP-2021 out through day 84, the efficacy assessment is continuing.
How would you sum up the potential of TP-2021?
These results indicate that TP-2021 implants can release drug above the therapeutic threshold in this mouse pruritus model for several months following a single treatment, and the expectation is that supra-therapeutic levels will continue to be maintained for a prolonged period. Difelikefalin, or Korsuva IV, administered three-times-a-week set the guidepost for the treatment of chronic pruritus in hemodialysis kidney disease patients. To date, efficacy of an oral formulation meant for treating other pruritic indications, such as atopic dermatitis, has not been demonstrated. Titan’s ProNeura-based TP-2021 implants present a viable solution for the delivery of sustained therapeutic levels of a peripheral kappa agonist for six months or longer for the treatment of chronic pruritus and related conditions. A single administration of the TP-2021-ProNeura implant could potentially abrogate the need for daily administration, even if a peptide with oral bioavailability can be successfully developed.
• • • • •
To connect with Titan Pharmaceuticals or any of the other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.







