
Anebulo Pharmaceuticals (NASDAQ:ANEB) is developing a novel antidote for cannabinoid intoxication and, longer term, other indications related to abuse and addiction.
“This is an unmet medical need in a large and growing market as legalization of cannabis for medical and recreational use is driving intoxications and visits to hospital emergency departments,” Dan Schneeberger, M.D., Anebulo’s CEO, says in an interview with BioTuesdays.
“No product is approved for this indication and our ANEB-001 drug candidate is furthest along in clinical testing as a Phase 2-ready asset to get underway in the fourth quarter of 2021,” he adds.
Anebulo raised $21-million in an IPO in May 2021 and suggests it has a cash runway to reach into a Phase 3 trial. However, it will require additional funding to complete the pivotal trial and potentially initiate commercialization.
Dr. Schneeberger explains that the central effects of tetrahydrocannabinol (THC) are mediated at the CB1 cannabinoid receptor in the brain. THC must bind to the CB1 receptor for a person to feel the cannabinoid’s intoxicating effects.
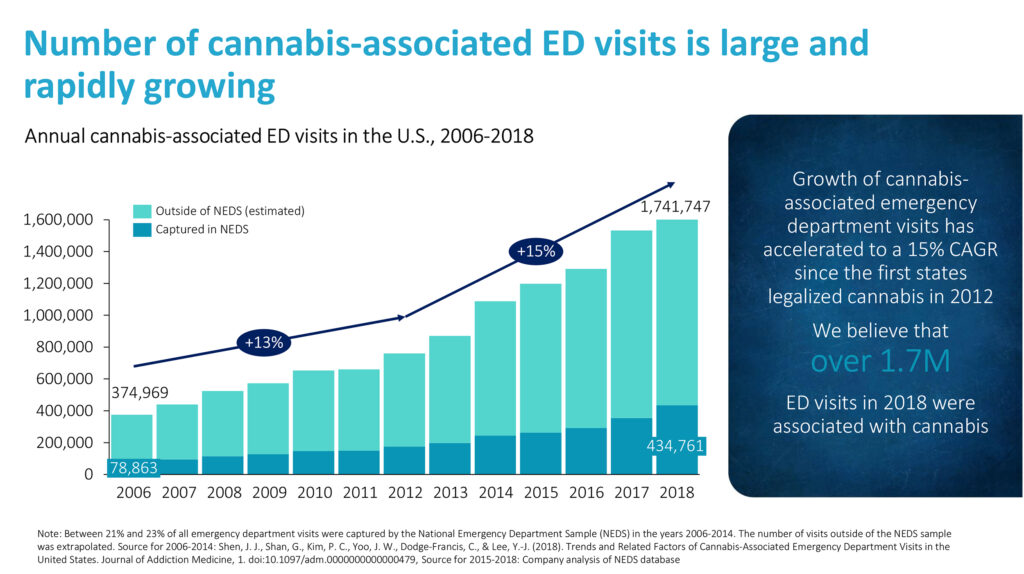
The number of cannabis-associated emergency department visits has increased by 15% annually since cannabis legalization began in the U.S. in 2012 and reached 1.7 million in 2018, according to an analysis of National Emergency Department Sample, a set of hospital-owned emergency department databases.
In a company-sponsored survey of 27 U.S. emergency room physicians in 2020, Dr. Schneeberger says on average, physicians saw 10.5 patients with cannabis intoxication a month, with a range of two-to-45. “We can treat patients within 40 minutes and discharge them within an hour.”
Marijuana legalization is increasing. Cannabis is legal for recreational use in 16 states and is legal for medical use in 35 states. Four states legalized recreational marijuana in 2020, followed by two additional states in 2021.
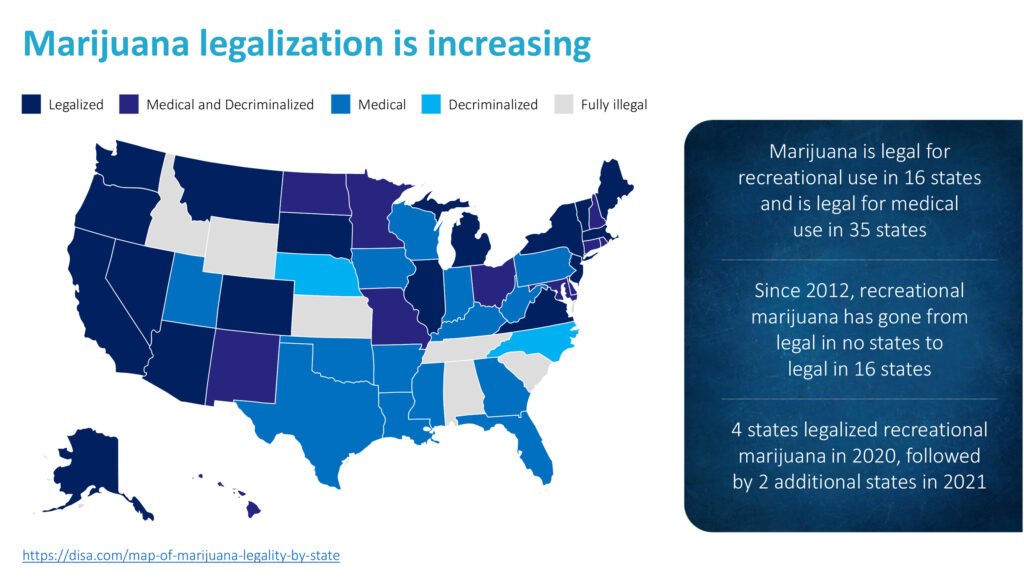
In addition, legalization is driving emergency department visits. A four-year study at the University of Colorado Hospital found that marijuana-related hospital visits tripled after Colorado became the first state to allow recreational sales in 2012, with two-to-three patients a day seeking aid with severe vomiting, anxiety and psychosis.
Dr. Schneeberger warns that the potency of cannabis edibles tends to be deceiving. “While consumers often approach cannabis edibles with the same serving size expectations as non-cannabis products, a cannabis candy bar may contain four times or more than a safe dose of THC, much higher than a consumer may expect.”
Children are particularly vulnerable to cannabis overdose because of brightly colored packaging of edibles and formulation into candies and other sweets, he adds.
According to Dr. Schneeberger, peak plasma THC concentrations occur in three-to-10 minutes with inhalation of cannabis, compared with two-to-four hours with ingestion. “Delayed reaction increases the risk of overdose with edibles, particularly for inexperienced users, and homemade edibles where dosing may be unexpectedly strong is another common cause of overdose.”
Synthetic cannabinoids, commonly referred to “spice” or “K2,” are the fastest growing class of psychoactive drugs worldwide and pose serious potential side effects, such as seizure, renal failures and death. “Synthetic cannabinoids are analogous to fentanyl for opioids insofar as they are more potent at the cannabinoid receptor than THC and will remain a problem for the foreseeable future,” Dr. Schneeberger predicts.
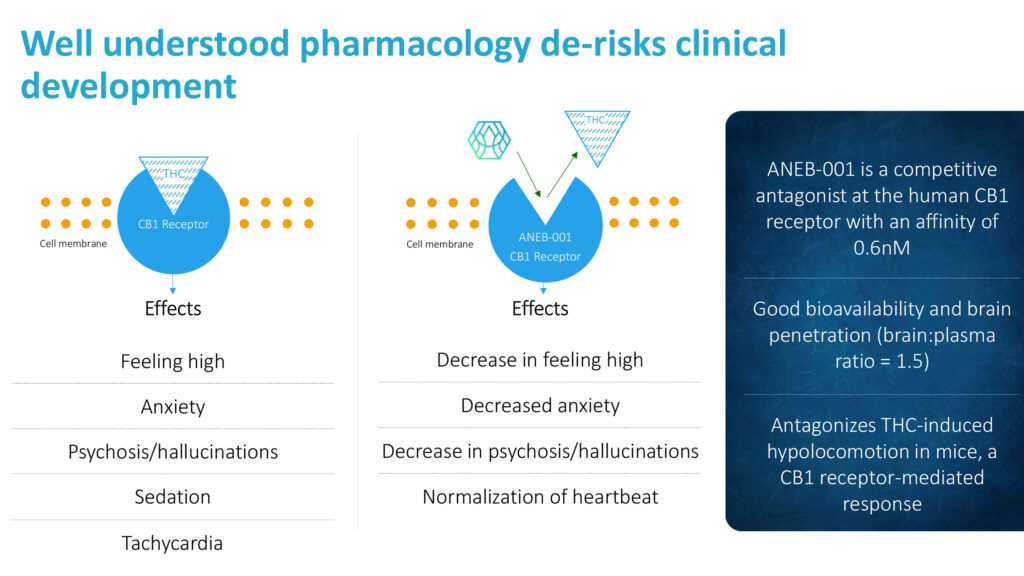
Anebulo’s solution, ANEB-001, has the potential to be administered as a pill, capsule or tablet and blocks the effect of THC at the CB1 receptor. “Our pharmacology is well understood and there is a low likelihood of drug-drug interactions, he points out, adding that the company is not aware of any “competing products to reverse the symptoms of cannabinoid intoxication that are further along in the development process than ANEB-001.”
Dr. Schneeberger contends that ANEB-001’s pharmacology is well understood and de-risks clinical development. Specifically, he suggests ANEB-001 decreases a high feeling, anxiety and psychosis/hallucinations, and normalizes heartbeat.
In an earlier study with 18 patients, ANEB-001 reached potentially therapeutic blood levels within 30 minutes, with no serious adverse events, and “achieved blood levels in excess of those predicted to be necessary for activity,” he adds.
Anebulo’s Phase 2 proof-of-concept trial design has been approved by the necessary regulator and ethics committee at one site in the Netherlands, with contract research organization drug capacity secured for November 2021.
The study, which is scheduled to begin in the fourth quarter this year, is expected to enroll 100 healthy volunteers, with each receiving 10 mg of THC orally, and then randomized to one of three doses of ANEB-001 or placebo. The company expects to announce results in the first half of 2022.
Dr. Schneeberger says Anebulo envisions developing and commercializing ANEB-001 in the U.S. and will explore other strategic collaborations to commercialize ANEB-101. The company also is exploring a non-oral formulations of ANEB-101 for cannabinoid hyperemesis syndrome, a condition following long-term use of marijuana, and other product candidates to treat cannabinoid and substance-related addiction.
“Cannabinoid overdose is becoming an increasingly widespread health issue and we aim to be at the forefront of this treatment,” he says.
• • • • •
To connect with Anebulo or any of the other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.







