
Closely-held ViaCyte, a company with two decades of experience, is utilizing pluripotent stem cell technology and novel delivery approaches to address multiple disease areas, with an initial focus and lead products designed to develop a functional cure for Type 1 diabetes.
“As a leading regenerative medicine company, we are developing novel cell replacement therapies based on two major technological advances: cell replacement therapies derived from pluripotent stem cells and medical device systems for cell encapsulation and implantation,” Michael Yang, president and CEO, says in an interview with BioTuesdays.
“ViaCyte was the first company to apply islet cell replacement therapies derived from pluripotent stem cells in clinical trials for the treatment of diabetes,” he contends.
The company’s two clinical-stage product candidates – PEC-Encap and PEC-Direct – are actively recruiting Type 1 diabetes patients for two, Phase 2 clinical trials. Data are expected in the second half of 2021 for PEC-Encap and first half of 2022 for PEC-Direct, with hopes to file biologics license applications in 2024 and 2025, respectively.
In addition, ViaCyte is expanding cell manufacturing capacity and capabilities to advance PEC-Direct, PEC-Encap, and its immune-evasive cell program for diabetes, PEC-QT, which is partnered with CRISPR Therapeutics, a leading gene-editing company, while also exploring research into new disease areas that may benefit from cell-based therapies, Mr. Yang contends.
“We have six years of experience manufacturing cGMP cells, devices and combination products,” he adds.
Mr. Yang, who moved into the executive suite in January 2021, says he was attracted to ViaCyte after examining its science, management team, manufacturing capabilities, IP position, partnerships and clinical breakthroughs to achieve the first islet cell replacement therapy for Type 1 diabetes.
“When I looked at the landscape, I was convinced we are at the leading edge of using cell therapy and regenerative medicine to address this important disease area.”
Mr. Yang has more than 20 years of senior industry experience, joining ViaCyte from Acadia Pharmaceuticals, where he was EVP and chief commercial officer. He also served as president of Johnson & Johnson’s Janssen Biotech unit, where he was responsible for building Janssen’s U.S. immunology business, generating more than $8-billion in annual revenue.
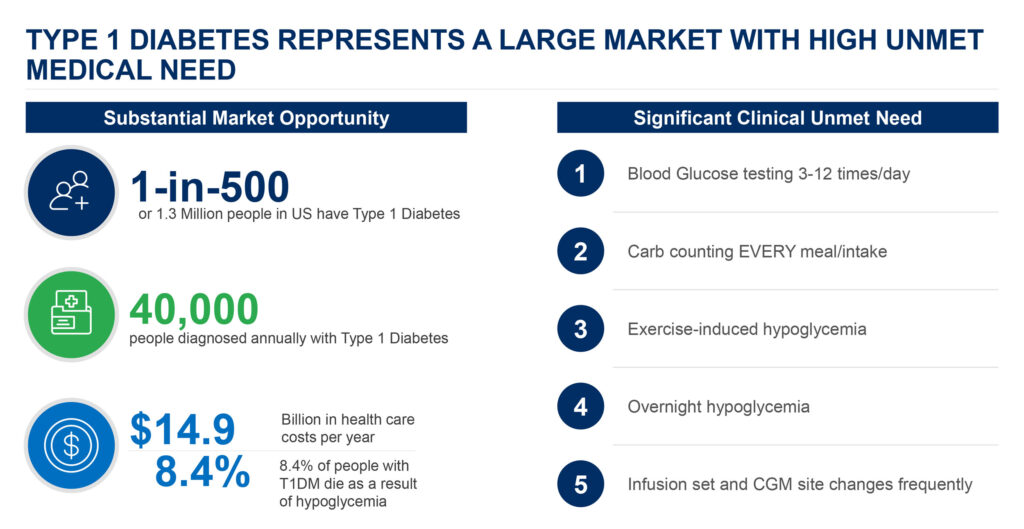
Some 1.3 million people in the U.S. have Type 1 diabetes, with 40,000 people diagnosed annually. In addition, 8.4% of people with the disease die each year as a result of hypoglycemia, where blood glucose levels drop dramatically below normal. Type 1 and Type 2 diabetes are expensive chronic conditions, resulting in billions of dollars in healthcare costs a year.
Mr. Yang explains that ViaCyte is focused on pluripotent stem cells because of their exceptional capacity to expand to large numbers and the company’s ability to direct their differentiation into various potentially therapeutic, specialized cell types, including pancreatic progenitor and beta cells for Type 1 diabetes.
The pancreatic progenitor cells are then contained in an encapsulation system, which is implanted subcutaneously. Mr. Yang says ViaCyte has shown that once implanted and successfully engrafted, the cells mature into beta cells that secrete insulin, alpha cells that release glucagon, and other cells of the human pancreas that naturally control blood glucose levels.
The company also has developed two device delivery platforms: open and closed, which contain the cell therapy while enabling oxygen and nutrients to flow into the device and insulin, glucagon, and other hormones to flow out. “There is no insulin pump today that produces glucagon, which helps raise blood glucose,” he adds.
The open device platform, which promotes direct vascularization of PEC-Direct but needs immune suppression drugs, is about three-quarters of the size of a bandage and is subcutaneous.
The closed encapsulation system uses a proprietary membrane jointly developed by ViaCyte and W.L Gore & Associates, a global materials science company, to improve engraftment, survival, and overall function of the implanted cells by mitigating the foreign body host response and providing protection from immune rejection, Mr. Yang says.
The closed encapsulation system also is designed to eliminate the need for immune suppression drugs commonly used with other transplants. The combined effort is being applied to the PEC-Encap product candidate.
“ViaCyte has demonstrated that when the cells successfully engraft, they produce both insulin and glucagon in Type 1 diabetes, a first in the field of cell therapy for diabetes,” Mr. Yang contends.
Mr. Yang points out that ViaCyte also is developing an immune-evasive cell line, PEC-QT, with CRISPR Therapeutics that is earmarked for the open device to avoid destruction by the patient’s immune system, potentially eliminating the need for immunosuppressants. The program currently is in preclinical testing, with plans to file a clinical trial application in the fourth quarter of 2021.
ViaCyte and CRISPR Therapeutics formed a partnership in 2018 to discover, develop, and commercialize gene-edited allogeneic stem cell-derived therapies, which could be a next-generation functional cure for all insulin-requiring Type 1 and Type 2 diabetes patients, he adds.
Mr. Yang said ViaCyte’s clinical program has shown human proof of concept. Nine of 33 patients with therapeutic device implants using the open device platform have shown improved time-in-range for normal blood glucose levels, histologic evidence of insulin and glucagon-secreting cells, and higher levels of C-peptide in the body, which provides evidence of graft-derived insulin production. All nine patients were C-peptide negative at enrollment, he adds.
With Type 1 diabetes, the pancreas makes little to no insulin, and little or no C-peptide, a biomarker used to help tell the difference between Type 1 and Type 2 diabetes.
Mr. Yang points out that a 22% increase in time-in-range equates to about 5.3 hours a day or 80 more days a year in euglycemic range.
In the closed device platform using PEC-Encap, 17 clinical subjects implanted with the Gore membrane device demonstrated that the encapsulation system reduced the foreign body response and improved engraftment, cell survival, and function. This program began a Phase 2 clinical trial in January with data expected in Q4 2021.
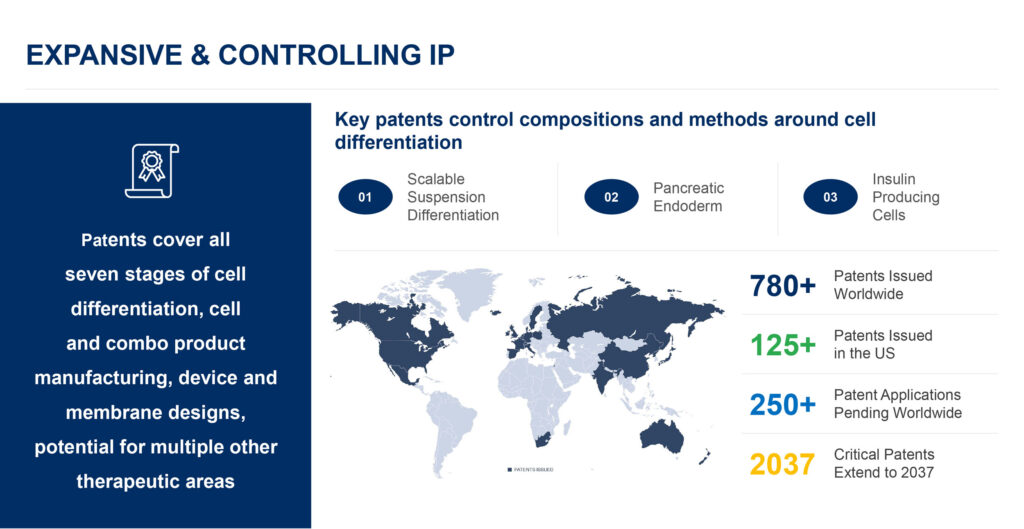
ViaCyte has more than 780 issued patents worldwide, of which more than 125 are in the U.S., with critical patents running until 2037. The company has some 250 patent applications pending worldwide.
Mr. Yang points out that ViaCyte’s patents cover all seven stages of cell differentiation, cell and combination product manufacturing, device and membrane designs, and potential for multiple other therapeutic areas where “our technology can make a difference for patients.”
Some areas for prioritization include chronic liver failure, hypoparathyroidism and hypothyroidism, and Type 2 diabetes, he suggests.
“Our foundation is built on deep expertise in translating science, manufacturing and device delivery into innovative solutions for patients,” Mr. Yang says. “We continue to be leaders in the field with our world class partnerships with CRISPR Therapeutics for development of a first-in-class immune evasive cell line and W. L. Gore for novel device and membrane technologies.”
• • • • •
To connect with ViaCyte or any of the other companies featured on BioTuesdays, send us an email at [email protected].






