
Achieve Life Sciences (NASDAQ:ACHV) began a pivotal trial in the fourth quarter of 2020 to evaluate cytisinicline, which is derived from the seeds of the laburnum tree, as a potential new treatment for smoking cessation and nicotine addiction in the U.S.
Cytisinicline has been sold in Central and Eastern Europe for more than two decades by Sopharma Group under the brand name TABEX to combat nicotine addiction. Achieve has an exclusive license to develop and commercialize cytisinicline outside the 17 European countries where TABEX is available.
“We have an unusual asset on our hands in that we’re in a Phase 3 program and have a tremendous amount of robust historical data behind us,” John Bencich, CEO, says in an interview with BioTuesdays.
More than 10,000 people have been treated in cytisinicline clinical trials outside the U.S., including three investigator-led Phase 3 trials with more than 2,700 patients. Those study results have been published in the New England Journal of Medicine. And Sopharma has a safety database exceeding 15 million cases.
Mr. Bencich says Achieve formed a collaboration with the NIH, which deemed cytisinicline to be a drug of national public health importance, and supplied $5-million for Achieve’s IND-enabling nonclinical studies which completed in in 2017.
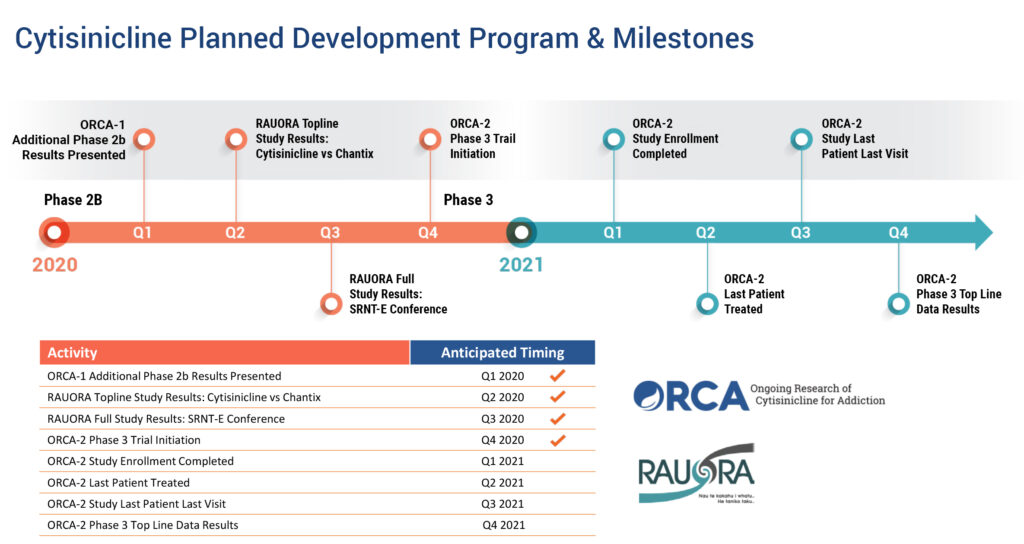
Since then, Achieve has completed a Phase 1/2 repeat-dose pharmacokinetics/ pharmacodynamic study; the Phase 2b ORCA-1 trial in 2019, showing statistically significant smoking quit rates; and supported an investigator-led head-to-head trial against Pfizer’s market-leading Chantix for nicotine addiction. The company hopes to report top line results from the ongoing Phase 3 ORCA-2 trial at the end of 2021.
“Cytisinicline has a well-differentiated product profile that includes a single and short course of treatment, compared with Chantix and Zyban, which are given on average for 12 weeks, and a highly selective mechanism of action, which contributes to improved tolerability,” Mr. Bencich contends.
In 2016, the FDA removed black box warnings on Chantix and Zyban about possible psychiatric side effects. In an earlier head-to-head study, cytisinicline had overall significantly fewer adverse events than Chantix, which showed significantly increased nausea, abnormal dreams, headache and insomnia. As a result, many Chantix patients fail to complete their full course of treatment.
Cigarette smoking rates have not declined in the past three years, with the number of smokers in the U.S. pegged at 34 million. Smoking and tobacco use is the leading cause of preventable death, with nearly 30% of all cancer deaths in the U.S. attributable to cigarette smoking. The global nicotine addiction market is valued at some $13-billion a year, with Chantix sales accounting for nearly $1-billion.
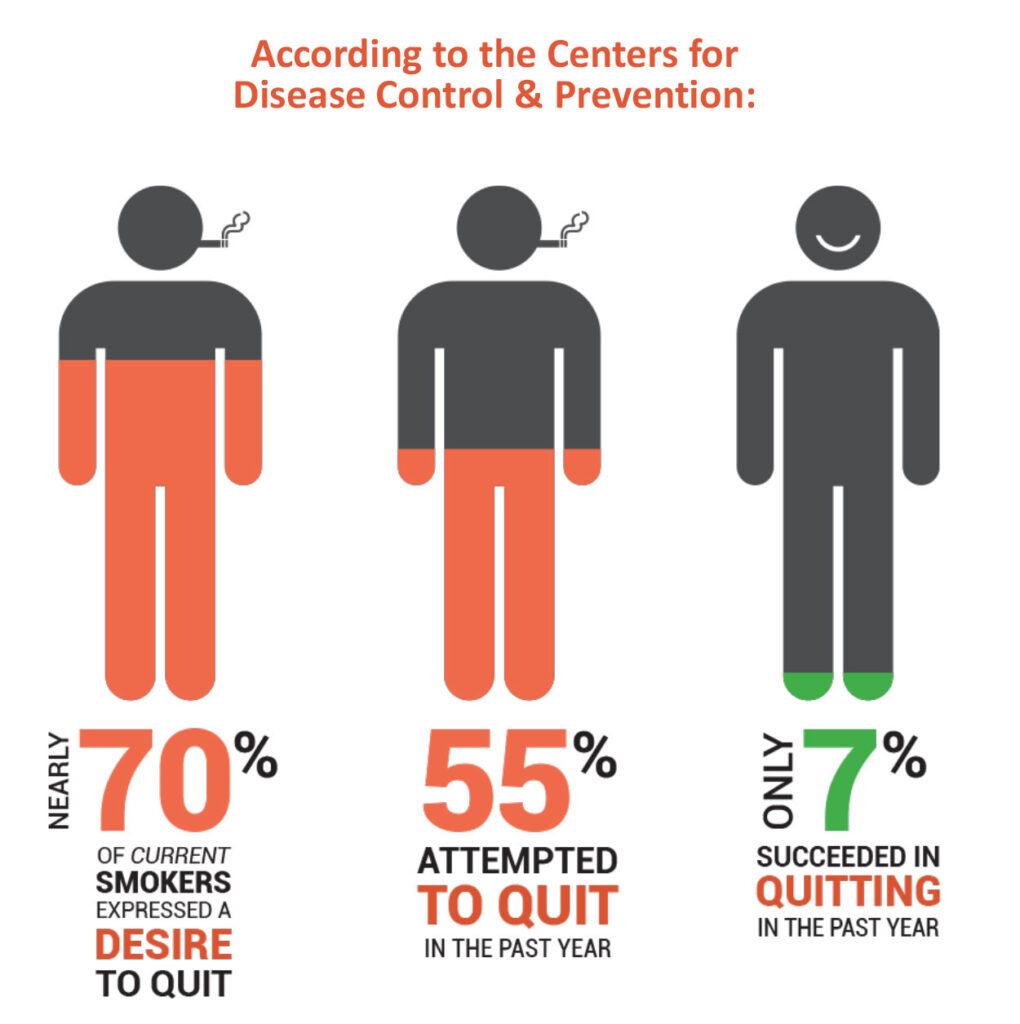
Smokers have been without a new treatment option for more than a decade, even as 70% of current smokers have expressed a desire to quit, 55% attempted to quit in the past year but only about 7% succeeded, according to the CDC. And up to 60% of quitters relapse in the first year because of the addictive nature of nicotine. Smokers make an estimated eight-to-11 attempts before quitting permanently.
On the other hand, e-cigarettes represent a growing market, with nearly 14 million adults in the U.S. vaping. Achieve has applied to the NIH for non-dilutive financing, and hopes to hear back by mid-year, about conducting a Phase 2 ORCA-V1 study of daily nicotine e-cigarette users who intend to try to quit vaping. There are no currently approved treatments specifically addressing vaping cessation.
Mr. Bencich explains that cytisinicline, which can’t be synthesized, specifically targets the alpha-4-beta-2 nicotine receptors in the brain. Working in two ways, cytisinicline binds to the receptor, partially stimulating dopamine release that reduces nicotine cravings and the severity of withdrawal symptoms. In addition, cytisinicline prevents receptor binding of nicotine to remove the nicotine-induced reward and satisfaction associated with smoking, he adds.
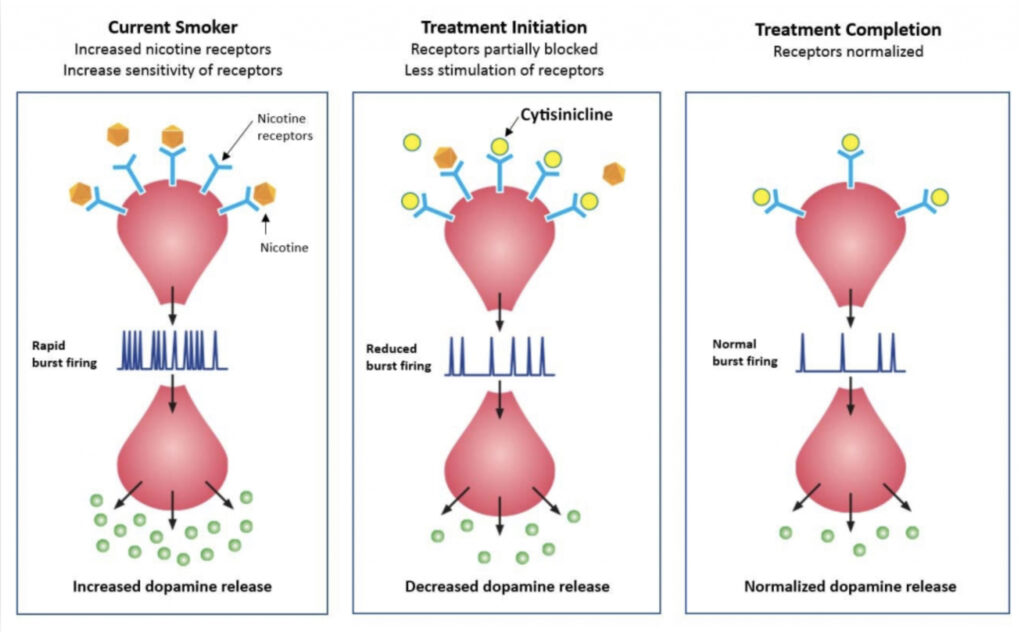
While Chantix also targets the alpha-4-beta-2 receptor, it binds to two other off-target receptors in the brain that could be responsible for its adverse event profile.
Among prior investigator-led clinical testing, Mr. Bencich points to the RAUORA trial of 679 subjects in New Zealand that compared cytisinicline with Chantix over 12 weeks. He says RAUORA’s primary endpoint of non-inferiority was met for cytisinicline with a “trend towards superior efficacy.” In addition, cytisinicline achieved higher quit rates among smokers and cytisinicline-treated subjects experienced a lower rate of adverse events, compared with Chantix.
Mr. Bencich says the company’s ORCA-1 Phase 2b trial enrolled 250 heavy smokers in six arms and was designed to evaluate safety and efficacy of 1.5 mg and 3 mg doses of cytisinicline, compared with placebo, over 25 days, with patients tracked out to eight weeks.
“The 3 mg dose administered three times a day was statistically significant to move forward into Phase 3 development,” he says. After four weeks, 54% of the patients receiving 3 mg of cytisinicline, three times daily, achieved biochemically confirmed quit rates, compared with 16% for placebo, which was “maintained over the subsequent four-week period,” he adds.
The ORCA-2 Phase 3 trial, which began last October, plans to enrol 750 adult smokers in three arms at 15 trial sites in the U.S. to determine the smoking cessation efficacy and safety profile of cytisinicline administered for either six-or-12 weeks, compared with placebo.
Arm A will treat 250 subjects for 12 weeks with placebo; arm B will treat 250 subjects with six weeks of cytisinicline, followed by six weeks of placebo; and arm C will treat 250 subjects with cytisinicline. Achieve hopes to complete enrollment in the current first quarter.
The primary outcome measure of success in the ORCA-2 trial is biochemically verified continuous abstinence during the last four weeks of treatment in the six- and 12-week cytisinicline treatment arms, compared with placebo. Secondary outcome measures will be conducted to assess continued abstinence rates through six months from the start of treatment.
As a patent-protected new chemical entity, cytisinicline would have five years of market exclusivity in the U.S., if approved, and up to 10 years in European countries where cytisinicline isn’t already approved. Sopharma has an exclusive agreement to supply Achieve with Phase 3 and potential commercial quantities of the active pharmaceutical ingredient.
Mr. Bencich says Achieve also is working with the University of Bristol to develop a derivative of cytisinicline for other indications, such as Alzheimer’s disease, schizophrenia and depression. “These would be out-license opportunities.”
• • • • •
To connect with Achieve Life Sciences or any of the other companies featured on BioTuesdays, send us an email at [email protected].






