
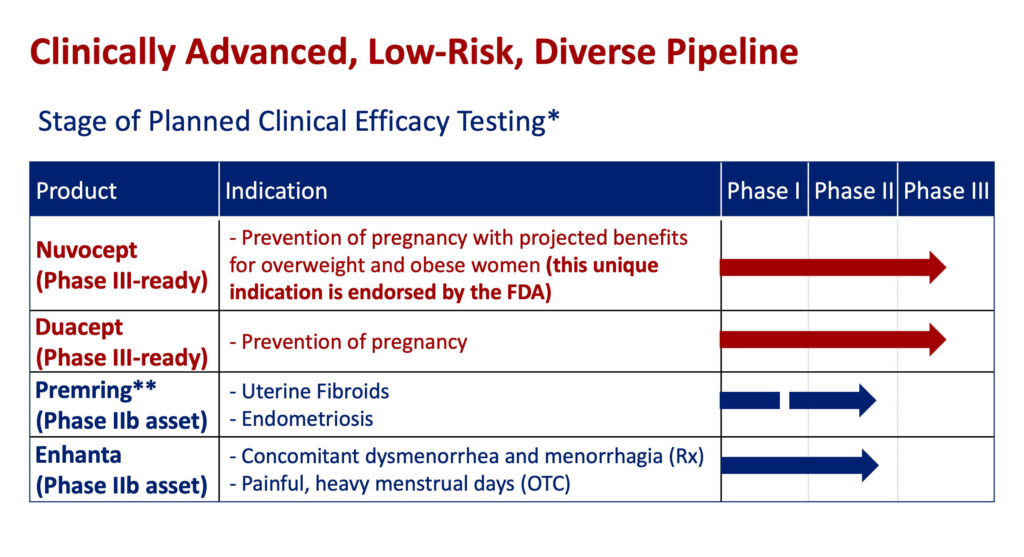
Closely-held ARSTAT Pharmaceuticals is advancing two patented Phase 3-ready assets: Nuvocept, the first and only oral contraceptive designed for overweight and obese women, and another contraceptive brand, Duacept, as a safer alternative to the best-selling European pill for normal-weight women.
The company’s portfolio also includes two additional transformational products: a first-in-class vaginal ring, Premring, for uterine fibroids and endometriosis, and a first-in-class single non-hormonal therapy, Enhanta, for painful and heavy menstrual periods.
“These are much-needed solutions in women’s health that offer substantially improved use of drugs with proven efficacy and safety,” Arkady Rubin, Ph.D., chief scientific officer and acting CEO, says in an interview with BioTuesdays. “The time, risks, and costs are markedly reduced because we are at an advanced R&D stage.”
Dr. Rubin figures the company’s portfolio of four drug candidates has a potential U.S. market of 50 million women, with projected peak sales of more than $2.8-billion a year. The worldwide addressable market for the portfolio could exceed 800 million women.
ARSTAT is in the process of raising $5-million ahead of a planned $45-million IPO in approximately one year. Among other things, the current financing round would be used to assemble an executive team, including a CEO, a board of directors, and business development and scientific advisors. Dr. Rubin plans to retain a senior R&D role within the company.
The IPO funding will enable the start of a Phase 3 clinical study of Nuvocept and Duacept in early 2022 and will support R&D through 2024. “Subsequent costs would be covered by the launches of Nuvocept and Duacept or out-licensing of Premring and Enhanta,” he adds.
With 30 years of pharmaceutical industry experience, mostly in women’s health, Dr. Rubin is a co-inventor of Ortho Tri-Cyclen Lo, a one-time leading oral contraceptive that had peak annual sales topping $500-million.
ARSTAT’s portfolio is protected by eight U.S. patents and a European patent, most of which are extendable to 2033-2034. All patents were developed by Dr. Rubin, using novel dosing regimens, such as for Nuvocept and Duacept; a novel drug delivery device for Premring; and Enhanta’s novel oral drug combination.
Nuvocept is formulated to address the efficacy and safety concerns of overweight and obese contraceptive pill users, of which there are six million women in the U.S. and 50 million women worldwide. “Marketed oral contraceptives do not work well for this population because of an increased risk of pregnancy and troublesome side effects,” Dr. Rubin contends.
Nuvocept is a novel multiphasic oral contraceptive with gradually increasing hormonal amounts and a constant progestin-to-estrogen ratio. A calibrated dose increase improves efficacy, while a constant ratio reduces unwanted side effects.
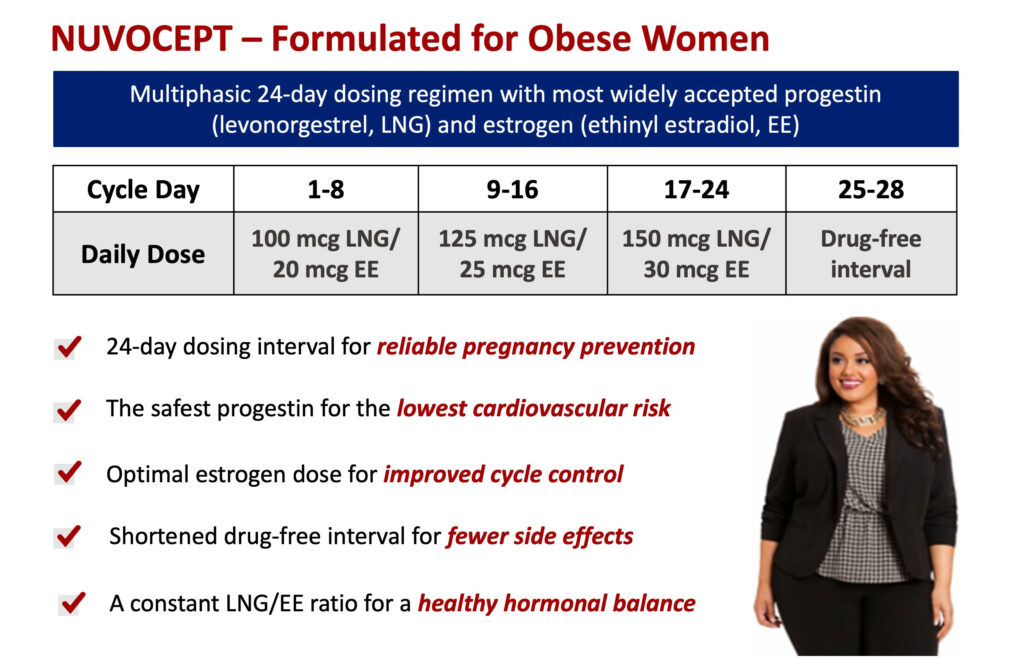
Nuvocept is the “first contraceptive product designated by the FDA for the exclusive clinical testing and labeling in women with a high body mass index,” he adds.
Dr. Rubin explains that modern contraceptives are not designed for obese women. Obesity alters the way hormones are absorbed, resulting in obese women receiving only 70% to 80% of an intended dose. “This results in an unacceptable protection from pregnancy with inevitable maternal and child health hazards.”
In addition, he says obesity is an independent risk factor for cardiovascular safety. The higher risk of venous thromboembolism makes pills with modern progestins unsuitable for obese women.
“Nuvocept utilizes progestin/estrogen combination and daily doses that are considered as a gold standard in cardiovascular safety with two-to-three times lower rates of serious side effects, compared with most competitors, ” he adds.
According to Dr. Rubin, Nuvocept’s claims regarding significantly reduced risk of unplanned pregnancy of up to three times are supported by “a well-established correlation between the components of the Nuvocept dosing regimen and the contraceptive efficacy metrics of approved products. These claims are further supported by pharmacokinetic and pharmacodynamic modeling of the suppression of ovulation, which is a key predictor of contraceptive efficacy, with an adjustment for the impact of the body weight on drug absorption.”
At a pre-IND meeting with the FDA, the efficacy and safety projections were accepted, and Nuvocept was cleared for Phase 3 dosing of more than 1,000 overweight and obese women. “For the first time in the history of regulated contraceptive research, the FDA has endorsed the clinical program and labeling fully dedicated to women with high body mass index,” he says. “Nuvocept has a low-risk path to reach the U.S. market in less than 3.5 years via an abbreviated 505(b)(2) NDA at R&D costs of less than $20-million.”
ARSTAT’s Duacept can be developed in parallel with Nuvocept, he says, and submitted under the same 505(b)(2) NDA. The FDA approved an abridged Phase 3 study design with estimated development costs of about $5-million. Duacept has the potential to reach 20% to 25% of the combined hormonal contraceptive market in the U.S.
Duacept is a patented mix of two popular pills, representing a safer alternative to a leading European brand. “As a combination of two marketed progestin/estrogen formulations, Duacept could be approved outside the U.S. with a limited, if any, amount of clinical work,” Dr. Rubin contends.
“Nuvocept and Duacept target different patient populations and will not compete against each other,” he adds.
ARSTAT plans to conduct a pre-IND meeting to finalize the clinical programs for its proprietary vaginal ring, Premring, to treat uterine fibroids and endometriosis. Premring utilizes a targeted, controlled delivery of a specific selective progesterone receptor modulator (SPRM). ARSTAT projects a Phase 2b clinical efficacy study in women with uterine fibroids starting in early 2022. Due to an overlap in desirable pharmacodynamic properties, this study may also support an endometriosis indication, he suggests.
He estimates Premring would require development costs of less than $8-million to reach Phase 3 testing stage in 2.5-to-three years.
Symptomatic uterine fibroids and endometriosis affect some 14 million women in the U.S. Existing hormonal treatments offer modest symptomatic relief often associated with severe menopausal side effects, including hot flashes and bone loss. “An appealing alternative is the regulation of progesterone receptors via SPRMs,” he says, adding that the major challenge is the right oral dose that is both efficacious and safe.
Premring offers optimal use of the most promising drug by delivering low doses directly to affected tissues, Premring addresses a major unmet medical need: a “safe and efficacious long-term therapy that significantly reduces the need for radical surgeries of at least 600,000 hysterectomies a year in the U.S. due to the targeted disorders,” he points out.
Dr. Rubin says there are multiple supporting sources for Premring, such as the performance of vaginal rings with the same class of drugs for contraceptive purposes.
“A nearly identical compound within the Premring dosing range was delivered intravaginally for one year for the prevention of pregnancy. Observed pharmacodynamic effects – far less painful and much lighter menstrual periods – are compelling enough to predict a successful outcome of pivotal endometriosis and uterine fibroids studies of Premring. Besides, the referenced ring was safe and well-tolerated. The same features are expected for Premring,” he contends.
ARSTAT is also developing prescription and over-the-counter formulations of Enhanta for some 25 million women in the U.S. who suffer from painful and heavy menstrual periods. Enhanta is a patented oral drug formulation that combines an NSAID and a low dose of tranexamic acid. Both Enhanta components are off-patent, available from multiple suppliers, and suitable for a 505(b)(2) NDA.
“Extensive clinical data support the efficacy and safety of this first-ever single non-hormonal therapy for a widespread disorder,” Dr. Rubin says, adding that Enhanta has the potential to reduce excessive menstrual blood loss by at least 40%, which meets U.S. and European clinical targets for the treatment of heavy menstrual bleeding.
Enhanta is Phase 2b-ready. He says an immediate move to Phase 3 could be considered if endorsed by the FDA and a confirmatory pre-IND meeting will be scheduled later this year. He estimates prescription R&D costs at less than $17-million, with an additional $5-million for the over-the-counter formulation in the U.S. and Europe. Potential FDA approval could be obtained in four years.
“This is a remarkable start-up with a clinically and commercially strong pipeline that will deliver value to stakeholders and many millions of women worldwide,” Dr. Rubin says.
Editor’s Note: This article does not constitute an offer to sell or the solicitation of an offer to buy any securities of ARSTAT Pharmaceuticals, and shall not constitute an offer, solicitation or sale of any security in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction.
• • • • •
To connect with ARSTAT or any of the other companies featured on BioTuesdays, send us an email at [email protected].






