PDS Biotechnology (NASDAQ: PDSB) expects to report preliminary data in the first or second quarter of 2021 from a collaboration with the NCI, evaluating its lead oncology product, PDS0101, for the treatment of advanced HPV-associated malignancies, with interim data from two other oncology programs possibly ready at the end of 2021.
“Our pipeline of immunotherapies is based on our Versamune platform, a potent T-cell-activating platform that has demonstrated efficacy without dose-limiting toxicity in a Phase 1 clinical trial,” Frank Bedu-Addo, Ph.D., president and CEO, says in an interview with BioTuesdays.
“Versamune also has the potential to work with a wide array of oncogenes and viral antigens,” he adds.
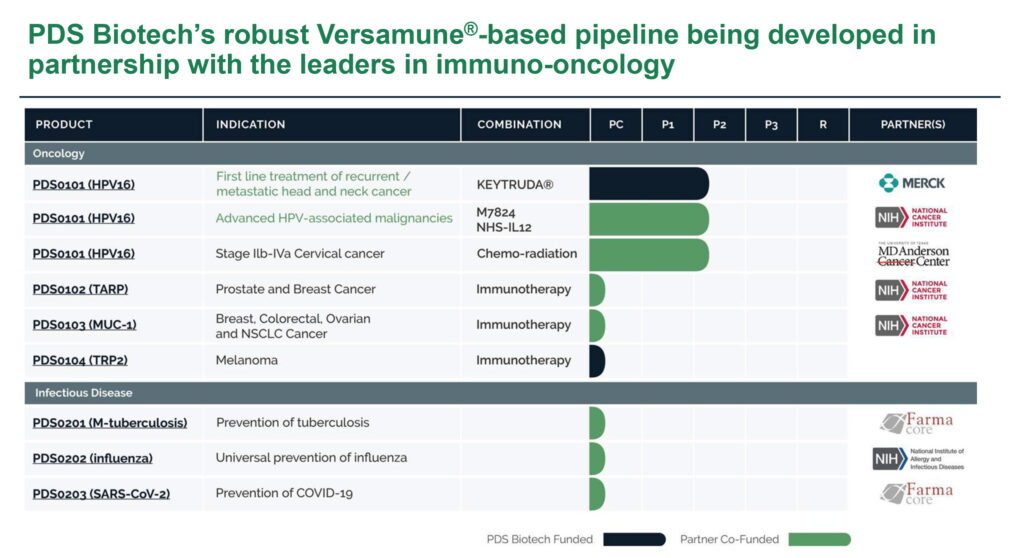
Besides the NCI partnership, PDS0101 is in a Phase 2 trial with Merck and its KEYTRUDA immunotherapy as a first-line treatment for recurrent/metastatic HPV-associated head and neck cancer, and a Phase 2 trial with the MD Anderson Cancer Center for all HPV types of advanced localized cervical cancer. All three programs are open label studies to determine the safety and efficacy of PDS0101.
“PDS0101 is designed to treat cancers caused by HPV,” including anal, cervical, penile, vaginal, vulvar and head and neck cancers, Dr. Bedu-Addo points out. Some 43,000 patients are diagnosed with HPV-associated cancers annually in the U.S., with the incidence rate growing despite increased use of HPV preventative vaccines.
Dr. Bedu-Addo explains that Versamune is comprised of a proprietary lipid and is combined with custom-designed antigens for a specific cancer type. The combination, which is delivered by subcutaneous injection, has been shown to “stimulate activation of Type 1 interferon genes and immunologic pathways associated with increased disease-fighting activity, such as MHC Class 1 and 2, resulting in the activation of CD8 killer T-cells and CD4 helper T-cells respectively.”
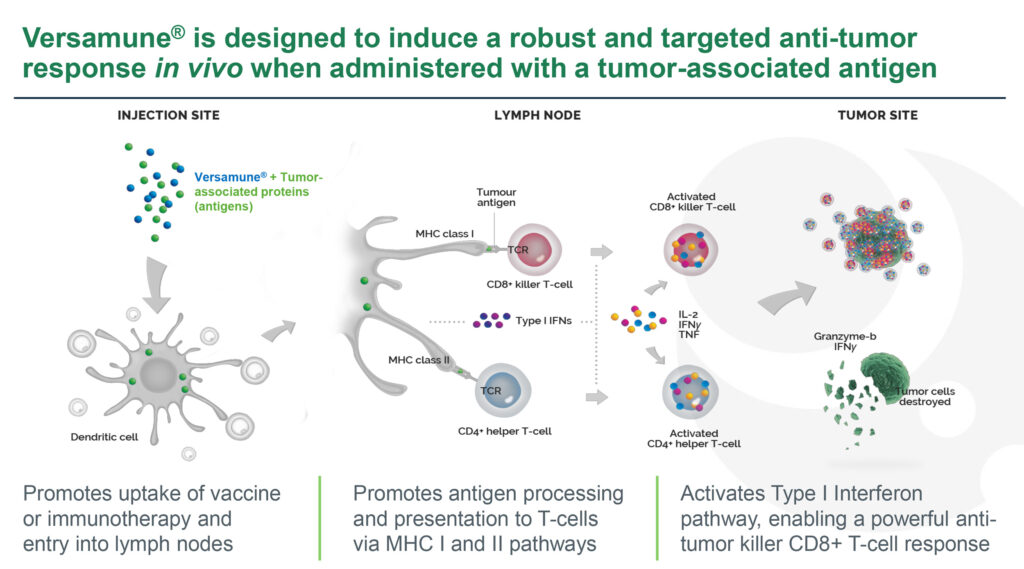
In addition, he notes that Versamune induces high levels of active CD8 killer T-cells and CD4 T-cells, with the ability to overcome tumor immune suppression. Versamune also induces long-term memory CD8 T-cells, as well as a low systemic toxicity risk.
“I like to say that not all T-cells are created equal and creating a T-cell response is not enough for a curative immune response,” he contends. “It is critical to induce the right type of killer T-cells, in the right quantity, with adequate killing potency. In our preclinical models, Versamune produced a more than 10-fold number of highly potent killer T-cells, compared with other T-cell technologies.”
According to Dr. Bedu-Addo, who has been CEO of PDS Biotech since its inception in 2006, says PDS0101 targets HPV16, the most prevalent strain of the human papillomavirus and is present in 70% or more of advanced HPV-associated cancers.
In an earlier Phase 1 study with PDS0101 in 12 patients with cervical intraepithelial neoplasia, a precancerous condition, and multiple strains of HPV, eight-of-10 patients had regression of their tumors, with 60% achieving complete tumor regression after one-to-three months. There were two non-responders and no dose-limiting toxicities.
The Phase 1 study also demonstrated a strong increase in circulating HPV disease-attacking T-cells as measured by several biomarkers, including interferon gamma and granzyme-b, Dr. Bedu-Addo points out.
“Based on our Phase 1 study, PDS0101 has shown strong potential for efficacy and safety as a monotherapy,” he adds. “This formed the basis of moving into two Phase 2 combination trials to enhance the clinical benefit of therapies already approved by the FDA for specific indications that are well tolerated, safe and effective.”
PDS Biotech’s most advanced Phase 2 program is its collaboration with the NCI, which has published preclinical data showing potent anti-tumor synergy and CD8 T-cell induction with PDS0101. Forty patients will be enrolled in the study, which began in June 2020, for the treatment of advanced HPV-associated cancers. The release of interim data are set for the first or second quarter of 2021, with final data in the second half of 2022.
Dr. Bedu-Addo points out that the NCI study is evaluating a triple combination of PDS0101 with a bi-functional checkpoint inhibitor and an antibody conjugated immuno-cytokine, which have demonstrated potential efficacy as monotherapies.
The objective of the Phase 2 study in conjunction with MD Anderson is to evaluate whether PDS0101 in combination with chemo-radiotherapy demonstrates improvement over standard-of-care of chemo-radiotherapy alone in 35 patients with advanced localized cervical cancer. The trial began in November 2020 and interim data are set for release at the end of 2021 or early 2022.
PDS Biotech is also collaborating with Merck in the Phase 2 VERSATILE-002 study in 96 subjects with recurrent/metastatic HPV16-associated head and neck cancer. The objective of the study, which also began in November 2020, is to determine whether the PDS0101-KEYTRUDA combination demonstrates improvement over standard-of-care with KEYTRUDA, a checkpoint inhibitor, alone. Interim data are expected at the end of 2021 or first half of 2022.
In addition to its three lead oncology programs, the company is developing three preclinical immunotherapy candidates for a variety of solid tumors, with two of those programs partnered with the NCI, as well as two Versamune-based infectious disease vaccines for COVID-19 and universal influenza.
“Today, four tumor antigens are being utilized with the Versamune platform, of the more than 75 identified by the industry, and our long-term strategy is to expand development of Versamune-based therapies through partnerships and licensing,” Dr. Bedu-Addo says.
• • • • •
To connect with PDS Biotech or any of the other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.