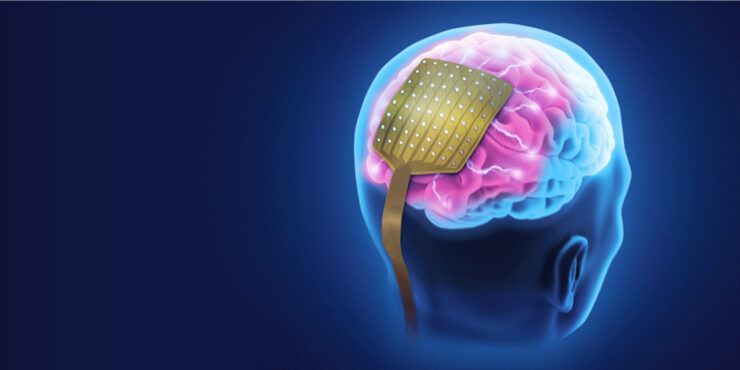
NeuroOne (OTCQB:NMTC) is developing high-definition, minimally invasive thin-film electrodes for the diagnosis and treatment of various neurological conditions, including epilepsy, Parkinson’s disease (PD) and chronic pain due to failed back surgeries.
“Our next-generation electrode technology has the potential to change the landscape of neurosurgical applications,” Dave Rosa, president and CEO, says in an interview with BioTuesdays. “It’s a true platform technology, based on thin-film, high-definition electrodes designed to record brain activity, ablate targeted problematic brain tissue and even stimulate parts of the nervous system for therapeutic purposes.”
NeuroOne’s Evo Cortical Electrode, which is placed on the surface of the brain, received FDA 510(k) clearance in December 2019. By the end of 2020, the company plans to complete a 510(k) submission to the FDA for its stereo electroencephalography (sEEG) Depth Electrode, which is designed for placement deeper within the brain.
‘We’re targeting motor neuron-related diseases such as epilepsy, PD and chronic pain, which are well-established markets,” he says. “But there is also great potential in the mental health space, where our technology might be useful to treat obsessive-compulsive disorder or depression, for example.”
Mr. Rosa explains that electrode technology was initially developed in the 1960s, but advances in the technology have not kept pace with those in other therapeutic areas: currently approved electrode technologies have limited resolution, require invasive surgeries for implantation and are expensive to manufacture, in part because they are assembled manually.
“Today’s electrodes are made in manufacturing clean rooms where gowned technicians place metal pellets onto a substrate and solder the leads in place,” he explains. “It’s not unusual for surgeons to schedule surgeries months in advance because they are waiting for product availability from vendors.”
To implant traditional electrodes, the surgeon removes the patient’s skullcap and places the electrode on the surface of the brain. Patients remain in hospital for up to two weeks, while the electrode records brain activity to identify areas of abnormality. A second surgery is required to remove the electrode as well as to treat the cause of disease.
In addition, older electrode technologies are bulkier and heavier, which can cause brain irritation and inflammation.
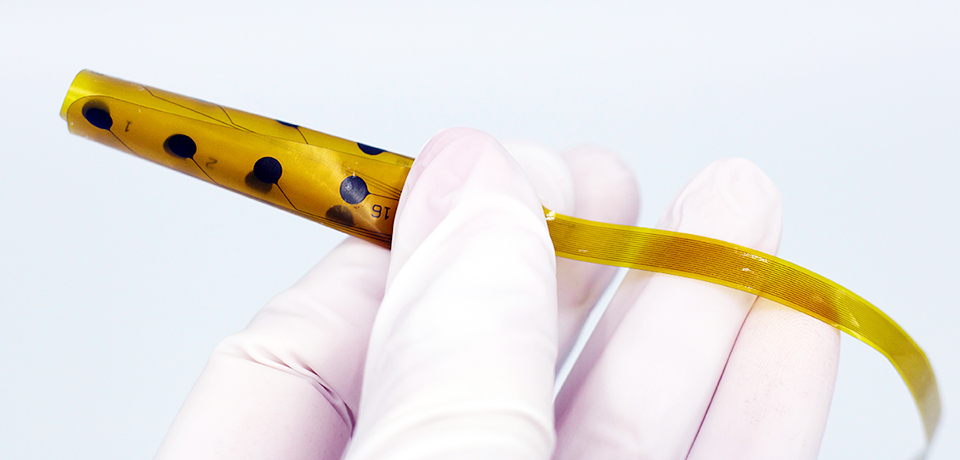
Evo Cortical Electrode
NeuroOne’s Evo Cortical Electrode technology is the result of a collaboration with the University of Madison-Wisconsin’s Alumni Research Foundation and the Mayo Foundation for Medical Education and Research. NeuroOne uses a fully automated manufacturing method called photolithography, which significantly reduces lead time and has the capability to increase the number of contacts that can be placed on a film.
“An important capability of this technology is higher resolution: we are able to scale, or downsize the size of the contacts, yet increase the number of contacts on the film,” he says. “One neurologist compared the different technologies using the analogy of television’s resolution in the 1960s versus today.”
In a study conducted at the Mayo Clinic, the Evo Cortical Electrode created less brain tissue inflammation, due to a reduced immunological response, at seven days post-implantation, compared with currently marketed electrodes. In addition to recording electrical activity, the electrode is also capable of stimulating brain tissue during a procedure for intraoperative mapping, which is conducted during surgeries to help surgeons identify and preserve essential functional tissue.

As NeuroOne’s electrodes are quite thin and flexible, the company is designing a tool to minimize the size of the skull portion that needs to be removed, which may make patients more willing to undergo the procedure. The FDA’s 510(k) clearance for NeuroOne’s Evo Cortical Electrode provides for recording, monitoring and stimulation for up to 30 days, after which the electrode needs to be removed.
NeuroOne is currently building inventory of Evo Cortical Electrodes for commercialization and is planning an initial targeted launch to select medical centers as soon as initial launch quantities are available.
NeuroOne’s sEEG Depth Electrode is designed to eliminate the need for removal of the skull plate altogether, while enabling access to deeper regions of the brain. Like the cortical electrode, the depth electrode is designed for recording, monitoring and stimulating brain tissue. However, this procedure typically uses a robot that drills tiny holes though the skull and into the brain, through which the electrode is guided and implanted.
Having completed animal feasibility studies with Dr. Jorge Gonzalez, director of the University of Pittsburgh Department of Neurological Surgery Epilepsy & Movement Disorders Program, NeuroOne plans to file a 510(k) submission for its sEEG Depth Electrode to the FDA by the end of 2020, with commercialization expected in early 2021.
The third device in NeuroOne’s development pipeline includes an sEEG Depth Electrode that would be capable of recording, monitoring and stimulating brain tissue; and ablating tumors or lesions that are associated with seizures. The combined recording, mapping and ablation capabilities enable diagnosis and treatment with a single procedure.
“The intention for our electrode design is that it can be implanted to record and identify the problematic brain tissue and can remain implanted until after ablative treatment is conducted. Leaving the device in place both eliminates the need for another surgery and supports more precise ablation than if the surgeon were to subsequently re-drill and re-insert an electrode for ablation only,” he says.
Mr. Rosa suggests other potential applications of NeuroOne’s technology, such as a deep brain stimulation device for the treatment of PD and epilepsy. Electrodes can also be used to treat pain that results from failed back surgeries, which represent about half of the spinal cord stimulation market.
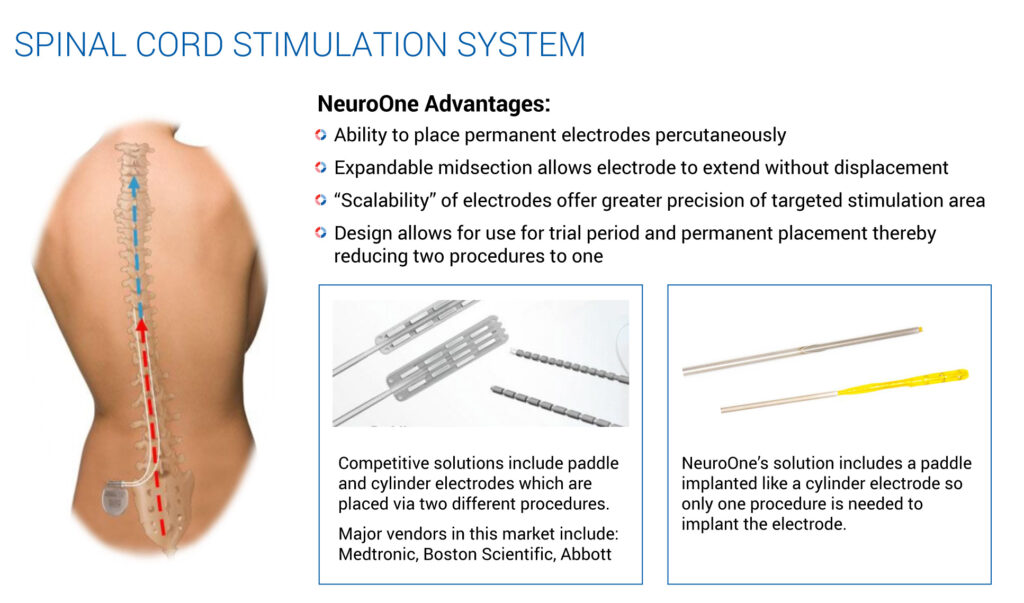
To address this market, NeuroOne is developing an electrode that could be placed percutaneously, much like an epidural injection. The company envisions temporarily implanting an electrode to determine whether the electrical stimulation improves the patient’s pain. Should the device prove effective after a seven-day trial period, the external power source can be permanently implanted, without the need to remove and re-implant another electrode.
Mr. Rosa points out that since these kinds of electrodes remain implanted for more than 30 days, they need to be designed and manufactured to last for years, and may require evaluation in clinical trials.
“Our technology has great applications in the indications we are currently working on, but there is a lot of interest in AI and brain-machine interfaces as well,” he says. “Thousands, or even millions of electrodes may need to be placed in the brain to say, enable someone who is paralyzed to walk again. There are already examples of patients guiding robotic limbs using their thoughts alone. This is what is referred to as a brain-machine interface. As the clinical indications become even more challenging, the ability to make much smaller contacts and electrodes will become a critical part of the solution.”
• • • • •
To connect with NeuroOne or any of the other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.