With FDA approval in October of its VMS+ 3.0 system, which turns 2D echocardiograms into MRI equivalent 3D images, Ventripoint Diagnostics
(TSXV:VPT, OTCQB:VPTDF) is gearing up for a major sales and marketing program in 2020.
“Working with a strong management and clinical team over the past few months, it is clear the company is now ready to achieve its full potential as we begin selling our new system to hospitals and cardiac centers worldwide,” Justin Leushner, president and CEO, says in an interview with BioTuesdays.
Mr. Leushner joined Ventripoint as president in September 2019 and moved into the executive suite earlier this month.
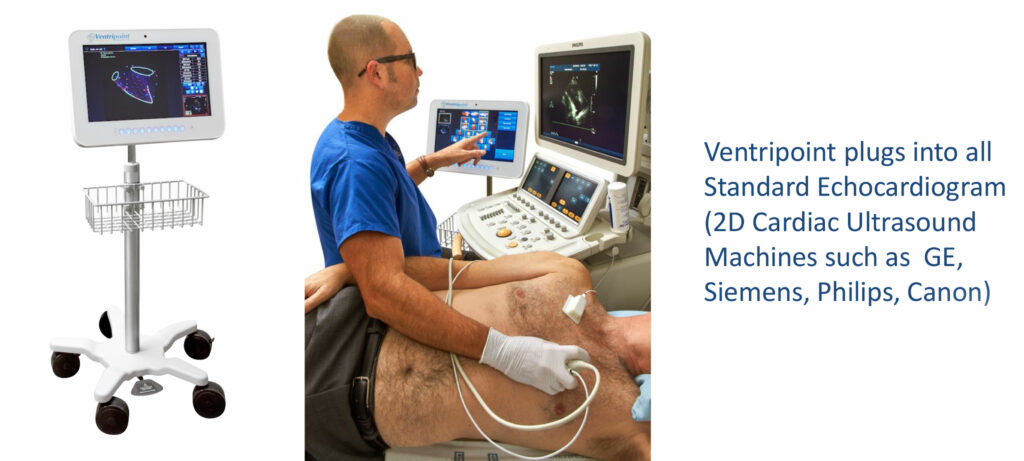
He explains that VMS+ 3.0 plugs into any echocardiogram machine, the most widely used cardiac imaging technology globally, or any 2D cardiac ultrasound, such as those made by GE, Siemens, Philips and Canon.
“The system uses a proprietary Knowledge Based Reconstruction, an artificial intelligence technology as defined by the regulators, to create 3D images of all four chambers of the heart and calculates volumes and ejection fractions with accuracy equivalent to MRI,” he adds. “Our device also is a cost-effective solution to an MRI.”
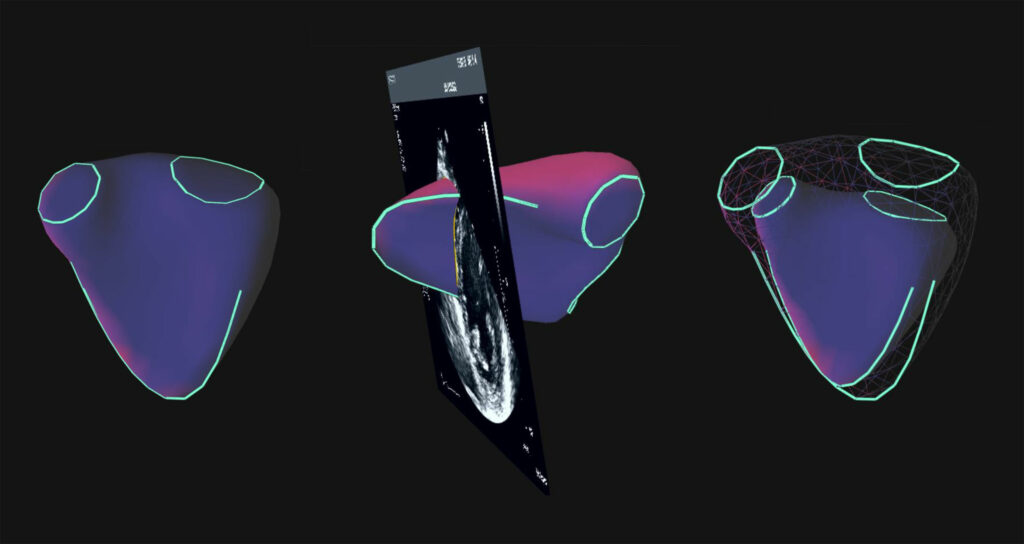
Noting that VMS+ 3.0 is also approved in Europe and Canada, he points out that the technology platform can be used to diagnosis heart disease in adults and children. “Accurate cardiac measurements are critical to assess heart disease, the leading cause of death worldwide.”
Mr. Leushner says Ventripoint’s VMS technology has been published in more than 60 peer-reviewed publications, which validated the technology and was part of the company’s regulatory evidence.
“Our short-term goal is to install 70 systems by the end of 2020, climbing to 400 by the end of year three, so system sales and recurring revenue from software and upgrades are compelling to potential partners,” he contends.
Ventripoint is employing direct sales in North America and the UK as well as distribution agreements in France, Spain and China under global sales director, Dave McPhedran, a 30-year medical device veteran and former sales director for Siemens, and Jim Graba, a registered diagnostic cardiac sonographer. The company also is in talks for additional distributors in South America, the Middle East and Asia.
“As our installed base grows, we would look to expand our sales and marketing efforts,” Mr. Leushner points out, adding that when the company demonstrates VMS+ 3.0 to clinicians, “they get the advantages immediately. One cardiologist compared our system to a microwave oven; once you use it, you can’t imagine not having one.”
According to Mr. Leushner, 2D echocardiograms do not obtain right ventricle volume and ejection fractions; do not provide a standard metric for all cardiac patients; and do not work on difficult to scan patients. “What these issues can lead to is a referral to a costly MRI procedure.”
However, MRIs have accessibility issues, compared with VMS+ 3.0, and many MRI patients require IV contrast dyes and sedation. In addition to standardized volumetric measurements of all four chambers of the heart, VMS+ 3.0 saves clinicians’ time by fitting into a standard echocardiogram exam.
Dr. Melissa Walton-Shirley recently highlighted some of the shortfalls of echocardiography in an article in Medscape, an online source of medical information for clinicians.
She writes that “poor-quality color flow Doppler from multimodality instruments can give the appearance of a bomb detonating in the left atrium, leading to an under- or overestimation of valvular regurgitation. Technical issues with the loading of images from offsite facilities can sabotage any hope of a well-estimated ejection fraction. Then there are reports with very little information: no mention of measurements, such as the left atrial dimension, no description of wall motion or wall thickness. In other words, we have work to do in the world of echocardiography.”
According to Mr. Leushner, there are upwards of some 40 million echocardiograms performed in North America each year, along with $1.4-billion spent on new echocardiogram machines. “There’s a large market out there for us to plug into.
Ventripoint initially is focusing on three markets, including pediatric and adult congenital heart disease patients; pulmonary hypertension and oncology.
Mr. Leushner points out that by adding VMS+ 3.0 to an echocardiogram, there is no need to send children for an MRI procedure, which can be cumbersome and often requires sedating a child, “with all the unpleasantness that implies for parents.”
And citing standard measurements obtained from VMS+ 3.0, adult congenital heart disease patients can be monitored regularly for changes in the state of their disease.
VMS+ 3.0 also can provide accurate, reproducible results of the right ventricle of pulmonary hypertensive patients, which can be difficult to measure but is an important piece of information for this large patient population, he adds.
“In oncology, chemotherapy is cardiotoxic, making tracking heart health in real time critical,” Mr. Leushner contends. “Our echo-enhancement device provides consistent, reproducible results across patients groups and different sites.”
Aiding in the potential adoption of the Ventripoint system is a CPT medical code for reimbursement from Medicare, Medicaid and private insurers of $80 for a 3D rendering, with interpretation and reporting, of computed tomography, magnetic resonance imaging, ultrasound, or another tomographic modality.
A 2D echocardiogram is currently reimbursed at a rate of $250. “When we add VMS+ 3.0, there is a significant cost advantage, compared with an MRI, which can cost between $1,000 and $2,000,” Mr. Leushner points out.
“All of which leads to the question of how many more patients would benefit from the utility of our add-on system?” he asks.
• • • • •
To connect with Ventripoint or any of the other companies featured on BioTuesdays, send us an email at [email protected].