TriSalus Life Sciences (NASDAQ:TLSI) is addressing the mechanical and biologic barriers within the tumor microenvironment (TME) that make it challenging to successfully treat liver and pancreatic tumors.
“High intratumoral pressure is a pervasive and underappreciated barrier to therapeutic success because it limits the delivery of standard-of-care therapies like chemoembolization, radioembolization and immunotherapy,” Steven Katz, MD, FACS, TriSalus’ chief medical officer, says in an interview with BioTuesdays.
Embolization involves using a material to block or decrease blood flow to tumors in effort to kill the cancer. Chemoembolization and radioembolization use materials that also deliver chemotherapy drugs and radioactive particles, respectively, to the area.
In 2020, TriSalus commercially launched the TriNav Infusion System, a Pressure-Enabled Drug Delivery (PEDD) technology used largely in patients with hepatocellular carcinoma (HCC) and colorectal cancer liver metastases. Designed to overcome the mechanical barriers to cancer treatment, the FDA-cleared system is inserted into an artery in the groin or arm and guided to the liver for the regional delivery of therapeutics.
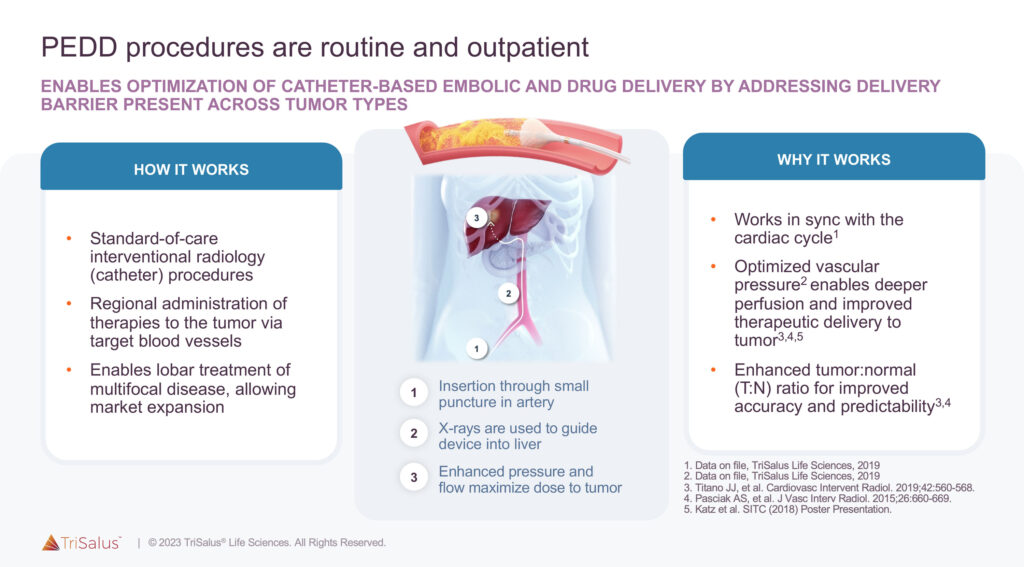
“The TriNav Infusion System has the unique SmartValve technology that opens and closes in sync with the patient’s cardiac cycle, resulting in significantly increased drug delivery to the tumor,” Dr. Katz contends.
In clinical studies of transarterial liver chemoembolization and radioembolization, the PEDD technology improved drug delivery by 60% and delivery accuracy by 33-to-90%, respectively, compared to standard catheter drug delivery.
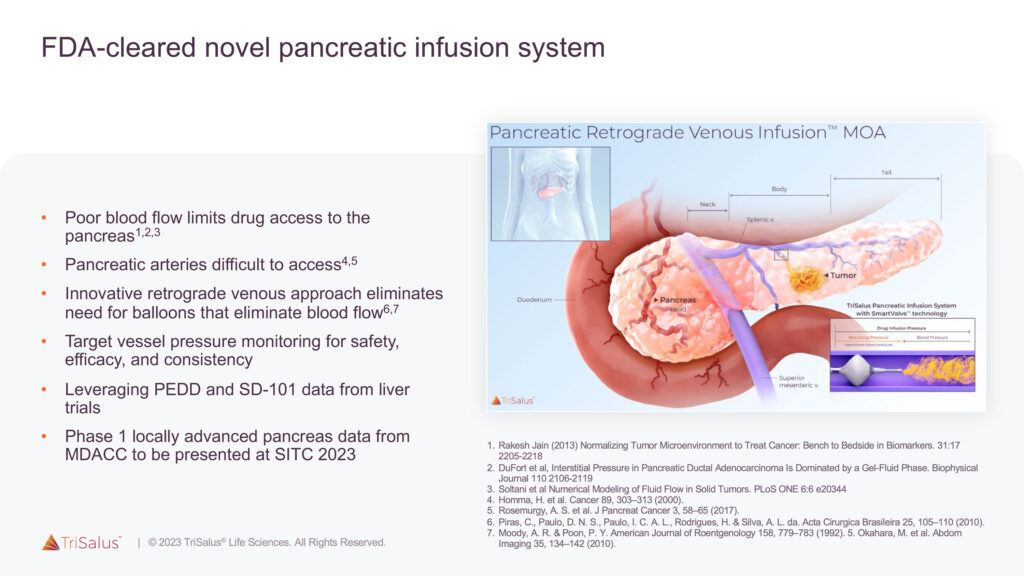
The company is also developing the TriSalus Infusion System, which uses a retrograde venous approach to deliver therapeutics to pancreatic tumors, which have poor blood flow and small arteries that are inaccessible for selective infusion. In a preclinical study, the FDA-cleared system improved chemotherapy delivery to the pancreas by 6.7-to-10.1 fold compared to systemic infusion.
“There are many opportunities to further expand our portfolio of drug delivery technologies,” Dr. Katz says. “In the first half of 2024, we are planning to launch our FDA-cleared TriNav Large Infusion System for larger blood vessels, while we continue to develop versions for smaller vessels and with integrated pressure sensing to enable more precise PEDD infusions to the liver or pancreas.”
The original TriSalus Infusion System is currently being evaluated in a Phase 1 clinical trial for the delivery of SD-101 in patients with locally advanced pancreatic cancer, TriSalus’ Class C toll-like receptor 9 (TLR9) agonist immunotherapy candidate. SD-101 is designed to overcome the biologic barrier of immunosuppression that challenges treatment with immunotherapies and checkpoint inhibitors (CPIs).
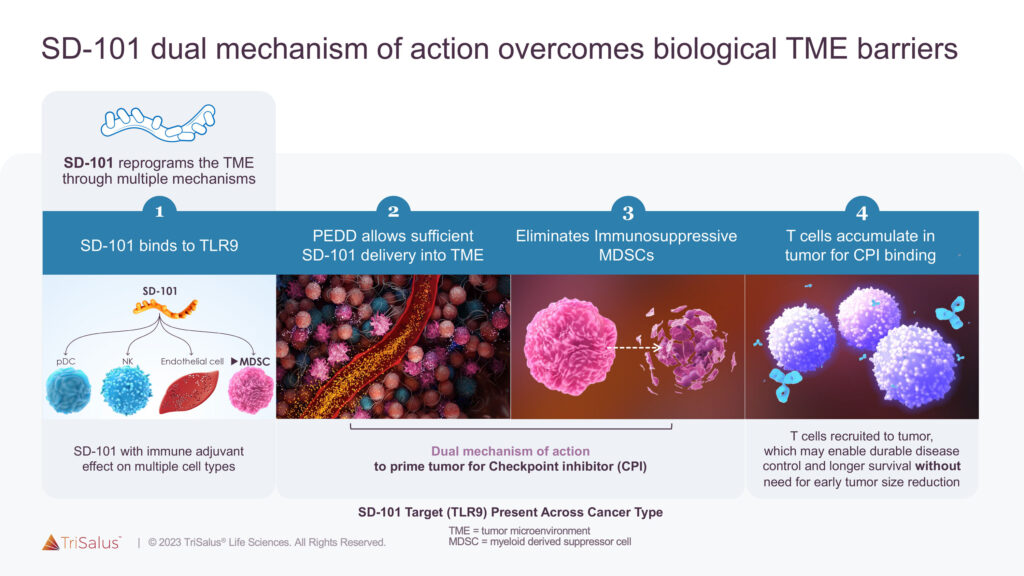
“SD-101 eliminates immunosuppressive myeloid-derived suppressor cells, or MDSCs, and recruits T cells to the tumor to enhance CPI activity and overcome the immunosuppressive TME,” Dr. Katz explains, adding that the ubiquity of TLR9 expression translates to a potentially broad application across multiple indications.
SD-101, combined with PEDD and CPIs, is currently being evaluated in multiple Phase 1 pressure-enabled regional immune-oncology, or PERIO, trials for the treatment of several liver and pancreatic cancer indications.
In partnership with MD Anderson Cancer Center (MDACC), TriSalus is studying the SD-101-PEDD-CPI combination for the treatment of metastatic uveal melanoma, an orphan drug indication. Data recently presented by MDACC’s Dr. Sapna Patel at the Society of Immunotherapy for Cancer meeting demonstrated that, when the optimal biologic dose of 2 mg SD-101 was combined with nivolumab, median progression-free survival was 11.7 months with an 81% disease control rate. Overall, the circulating tumor DNA clearance rate was 59%.
“The results exceeded our expectations,” Dr. Katz points out. “The study showed encouraging immunologic responses, including T cell activation with depletion of MDSCs and regulatory cells, as well as a favorable safety profile.”
TriSalus aims to confirm the optimal SD-101 dose for this indication in the first half of next year. The company is also developing the PEDD technology and SD-101 for the treatment of HCC, locally advanced pancreatic ductal adenocarcinoma (PDAC), and intrahepatic cholangiocarcinoma (ICC), another orphan drug indication.
In the first half of 2024, TriSalus plans to announce Phase 1b PERIO data for HCC and ICC; initiate a cohort evaluating SD-101 combined with radioembolization in HCC patients; and launch a Phase 1b trial for locally advanced PDAC. The company expects to report Phase 1 PERIO data in the second half of 2024.
Having launched the TriNav Infusion System in 2020 with sales of some $5-million that year, the company is forecasting sales to reach $19-million in 2023. TriSalus’ chief financial officer, Sean Murphy, says that the company has the capacity to grow to a $150-million sales range with existing capital. “We recently approached gross margins of 90% on TriNav Infusion System sales and expect to achieve cash-flow positivity by the end of 2024,” he adds.
With a current estimated market share of less than 10%, TriSalus recently expanded its sales team from 10 to 21 representatives, with plans to add nine more individuals in anticipation of further growth.
“The chemoembolization and radioembolization market alone has the potential to generate up to $400-million in sales,” says Mr. Murphy. “Our focus now is on highlighting the potential of our PEDD technology and added the benefits of SD-101 over existing methods, while increasing product adoption among practitioners.”
• • • • •
To connect with TriSalus Life Sciences or any other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.