By Melane Sampson

IMUNON (NASDAQ:IMNN) is advancing a distinctive approach to treating of some of the world’s most challenging diseases, leveraging a portfolio of proprietary immunology and DNA-based platforms that harness the body’s natural mechanisms to generate safe, effective, and durable responses.
“We are developing non-viral DNA technology across various modalities, beginning with a novel gene therapy, TheraPlas, which also serves as an immunotherapy designed to enable gene-based delivery of cytokines and other therapeutic proteins for the treatment of solid tumors, particularly where an immunological approach shows promise,” Stacy Lindborg, Ph.D., president, CEO, and board director of IMUNON, says in an interview with BioTuesdays.
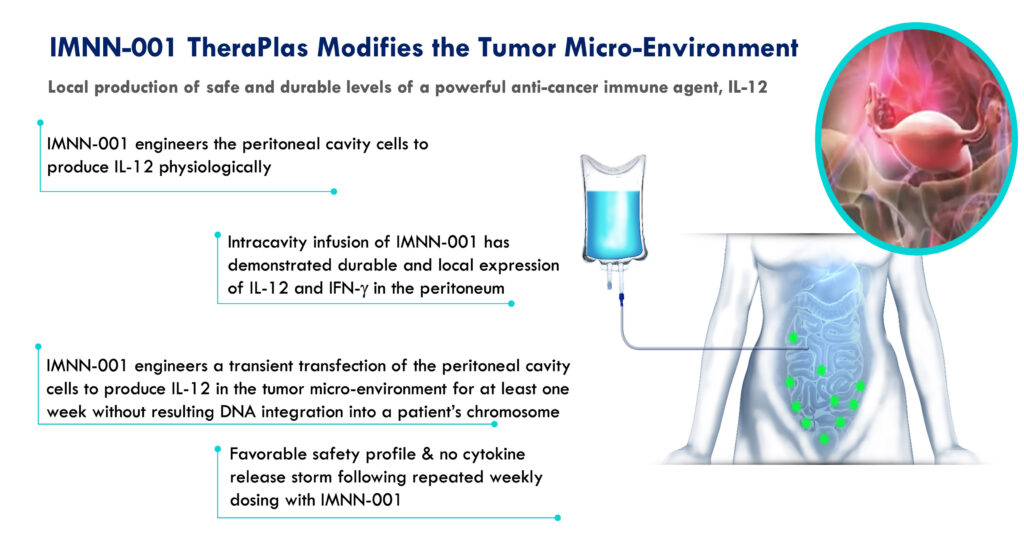
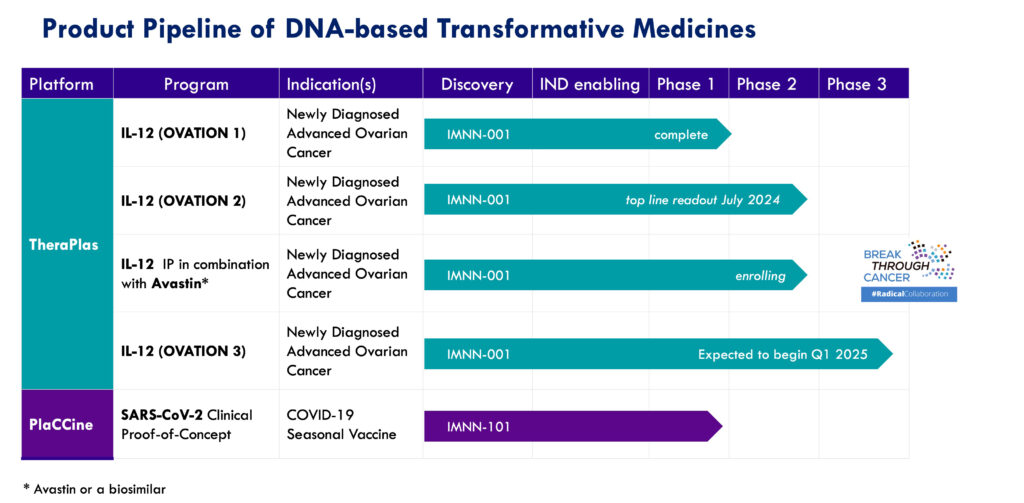
The company’s lead clinical program, IMNN-001, designed using its TheraPlas platform technology, is a DNA-based immunotherapy targeting advanced epithelial ovarian cancer (EOC). The program recently completed Phase 2 development. IMNN-001 functions by instructing the body to produce sustained levels of potent, cancer-fighting molecules, such as interleukin-12 (IL-12) and interferon gamma, at the tumor site. IL-12, a key cytokine, plays a critical role in stimulating anticancer immunity by promoting T-lymphocyte and natural killer cell proliferation.
Dr. Lindborg explains that EOC is hard to diagnose due to vague symptoms often mistaken for other conditions, resulting in 80% of women being newly diagnosed at stage III or IV. In the U.S. alone, approximately 20,000 women are diagnosed annually, and roughly 13,000 deaths will occur due to advanced ovarian cancer.
“Ovarian cancer in newly diagnosed patients is the optimal setting for immunotherapy, and IMNN-001 has the potential to become the first immunotherapy that is effective for these patients,” Dr. Lindborg contends. “This product is revolutionary, and as our Phase 2 data show, it has the potential to transform the standard-of-care (SOC) in advanced ovarian cancer, and beyond.”
According to Dr. Lindborg, EOC ranks as one of the deadliest gynecological malignancies, and the fifth leading cause of death in women. “There is an urgent medical need in this patient population. Despite significant investments made over the years, improvements in overall survival have remained elusive, and frontline SOC treatment for newly diagnosed patients remains unchanged for 25 years—a platinum-based chemotherapy regimen.”
EOC is characterized by the dissemination of tumors in the peritoneal cavity, with a 75% recurrence rate in patients with stage III or IV after SOC. Given the five-year survival rates for stage III and stage IV patients range from only 41% and 20%, respectively, Dr. Lindborg emphasizes the need for therapies that not only reduce recurrence but also enhance overall survival. The peritoneal cavity of advanced ovarian cancer patients contains the primary tumor environment and represents an ideal target for regional immune modulation, providing an opportunity for innovative therapeutic strategies.
“Frontline treatment of ovarian cancer is an intentional part of our strategy,” Dr. Lindborg notes. “Many companies focus on populations that have already responded to upfront chemotherapy, in maintenance or second-line settings, and are therefore easier to treat populations compared to a newly diagnosed patient population. However, we believe the mechanism of action of our product maximizes its potential in frontline treatment. When there is an urgent medical need paired with an innovative treatment, it often leads to a significant business opportunity, which is certainly the case with IMNN-001.”
IMUNON recently announced the outcome of its end-of-Phase 2 meeting with the FDA, paving the way for IMNN-001 to enter a Phase 3 pivotal study. “This marks a critical milestone for our IMNN-001 clinical program, and we’re pleased that we are aligned with FDA on our goals and the path to our vision to address the unmet needs in ovarian cancer,” Dr. Lindborg says. “The highly favorable IMNN-001 benefit-risk profile from our Phase 2 OVATION 2 Study, including positive outcome data presented at the SITC Annual Meeting, are very exciting.”
IMNN-001 is the first immunotherapy to achieve a clinically meaningful response in ovarian cancer, including benefits in both progression-free and overall survival in frontline treatment. “Our goal is to replicate these remarkable results in a Phase 3 trial, which would be transformative for the current SOC, substantially improving overall survival and offering hope to thousands of women with advanced ovarian cancer who continue to experience disease progression,” Dr. Lindborg says.
The company remains on track to initiate its 500-patient Phase 3 trial in early 2025.
IMUNON’s second platform, PlaCCine, is a novel DNA plasmid vaccine designed to elicit strong immune responses to a viral antigen. Dr. Lindborg highlights that PlaCCine emerged through a modification of IMUNON’s IL-12 TheraPlas immunotherapy platform. “We have the scientific depth in our company to take our scientifically advanced, novel platform and reposition it.”
Dr. Lindborg also emphasizes the urgent global need here, noting that more than one million people die annually from vaccine-preventable diseases. “IMUNON’s clinical development of nucleic acid-based therapies and vaccines for infectious diseases and cancer holds the potential to address some of the world’s most significant health challenges.”
Looking ahead, Dr. Lindborg underscores IMUNON’s focus on leveraging its advanced technologies to bring therapeutic products to market through partnerships and strategic licensing, specifically highlighting this strategy for PlaCCine.
“Our team’s rich global experience in clinical trials and regulatory engagement across multiple countries gives us an advantage in delivering innovative solutions for difficult-to-treat diseases,” Dr. Lindborg concludes. “We are well-positioned to capitalize on our strengths and technology synergies to bring life-changing treatments to patients in need.”
• • • • •
To connect with IMUNON or any other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.