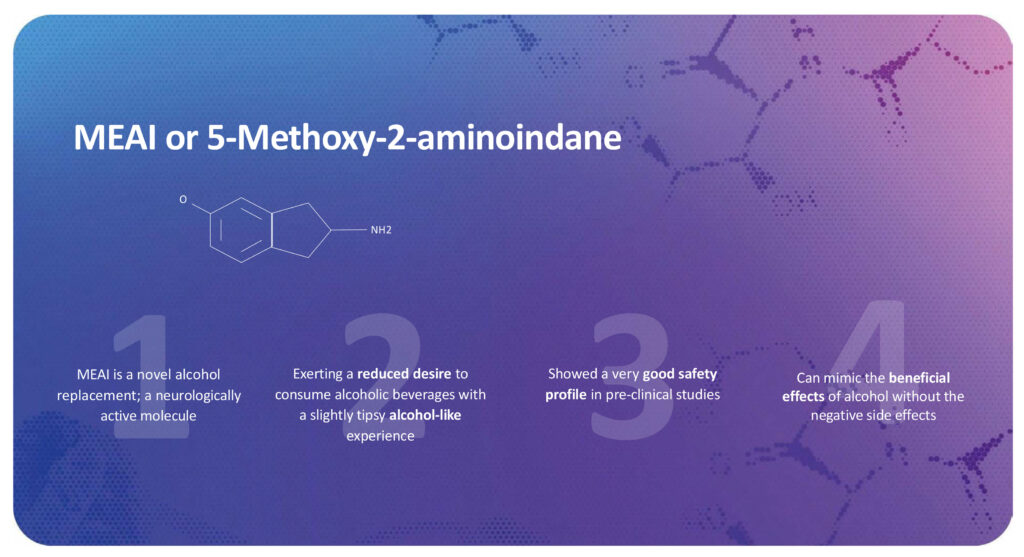
Clearmind Medicine (NASDAQ: CMND; FSE: CWY0) is discovering and developing safe, novel psychedelic-derived therapeutic candidates with its new psychoactive molecule, 5-Methoxy-2-aminoindane (MEAI), to address widespread and underserved, critical health challenges such as alcohol use disorder (AUD), binge eating, addiction, and depression, which affect millions globally.
“Our first indication is AUD, and MEAI holds the promise to break the binge-drinking vicious cycle by potentially innervating neural pathways, such as 5-HT1A, leading to sensible behavior,” Adi Zuloff-Shani, Ph.D., CEO of Clearmind says in an interview with BioTuesdays, alongside Mark Haden, VP of business development at Clearmind.
Although Clearmind is currently focused on AUD, literature suggests that 5-HT1A receptors are associated with controlling craving behavior across the board, indicating that MEAI may have a wide range of applications beyond binge drinking, she adds.

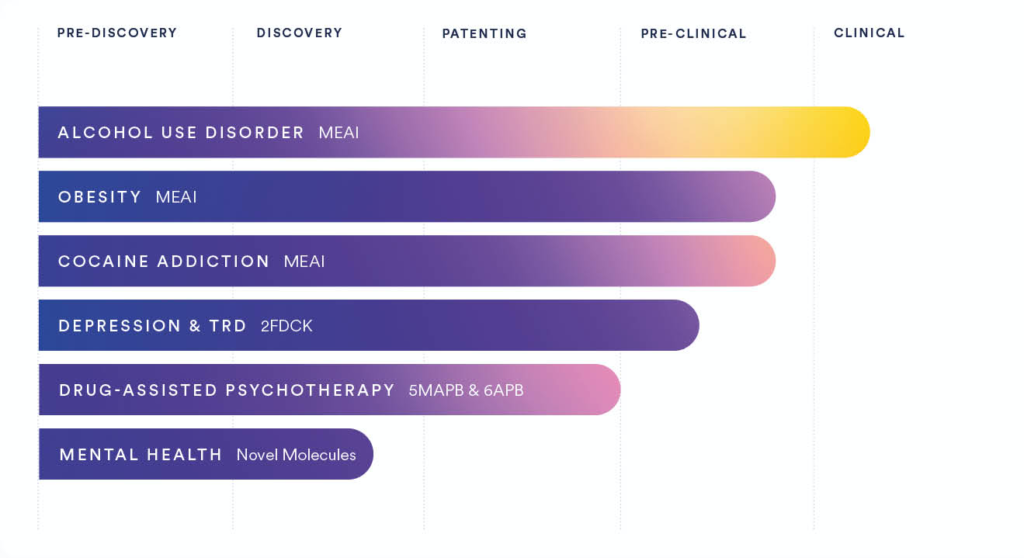
In addition to its clinical-stage AUD indication, Clearmind’s proprietary treatment candidates include therapies for obesity, cocaine addiction, depression, drug-assisted psychotherapy, and mental health.
Dr. Zuloff-Shani emphasizes that studies have demonstrated MEAI’s interaction with serotonergic receptors 5-HT1a and 5-HT2a, which play a key role in regulating alcohol intake, reward, preference, and dependence. MEAI also interacts with alpha-2-adrenergic receptors plasma membrane monoamine transporters for dopamine, norepinephrine, and serotonin. These receptors and transporters are believed to mediate alcohol-drinking behavior and could constitute important molecular targets for interventions targeting drugs subject to abuse, such as alcohol.
“MEAI is a psychoactive molecule that produces a euphoric alcohol-like experience and reduces the desire to consume alcohol. In pre-clinical studies, MEAI demonstrated a high safety profile and a promising efficacy, with the potential to change the lives of millions who struggle with drinking alcohol in moderation,” Dr. Zuloff-Shani contends.
More than 18 million Americans struggle with AUD. Each year 95,000 preventable deaths in the U.S., and three million globally, are attributed to alcohol misuse. Excessive alcohol use burdens the U.S. healthcare system by $250 billion, annually.
“MEAI was invented in the 1970s and has been used as a recreational substance. This underground use generated anecdotal data, so we are essentially reverse-engineering it because, while we now have pre-clinical evidence supporting MEAI for AUD, we also have extensive anecdotal evidence from past human experiences with MEAI,” Dr. Zuloff-Shani says.
The company holds many intellectual (IP) holdings of MEAI patents, with a growing IP portfolio of 19 utility patent families including 28 granted patents. Clearmind intends to seek additional patents for its compounds and is interested in the acquisition of supplementary IPs to expand its portfolio.
Mr. Haden points out that existing treatments for AUD, such as Antabuse and Naltrexone, are unattractive because they reduce the pleasure of drinking alcohol and often cause negative side effects, such as nausea and vomiting. In contrast, MEAI is a binge-mitigating substance that delivers a euphoric alcohol-like experience, culminating in a sensation that reduces the desire for further alcoholic beverages.
“Unlike traditional AUD treatments, MEAI actually feels good,” he explains. “It gives you a positive, pleasurable experience because it feels like alcohol. As you continue to consume it, the feeling transitions into a mildly euphoric, warm and fuzzy feeling of contentment, and then you hit the wall, you’re done. It gives the user a liquid satiation experience—a signal to the brain that says I’ve had enough.”
To illustrate the MEAI experience, Mr. Haden compares it to enjoying chocolate cheesecake. “Imagine having consumed two pieces of chocolate-covered, goopy cheesecake and someone puts a third piece in front of you. Not in your wildest dreams could you even think of touching it, because you are satiated—you’re just finished with cheesecake. MEAI offers a similar sense of satiation that kicks in after it has given you a pleasurable experience.”
Dr. Zuloff-Shani notes that the company is pursuing a dual approach for MEAI in AUD. This includes its clinical-stage oral capsule, CMND-100, which is consumed with alcohol and has the potential to control craving behavior, and its MEAI-based beverage aimed at delivering an alcohol-like experience without alcohol.
“As a pharmaceutical company, we are at the Phase I/IIa clinical stage with our MEAI-based CMND-100 oral capsule. Our primary endpoint is to determine the tolerable dose and characterize the safety, pharmacokinetics, and pharmacodynamics of single and repeated doses. The secondary goal is to evaluate CMND-100’s preliminary efficacy in reducing drinking patterns and cravings in individuals with moderate-to-severe AUD,” she outlines.
In July, 2024, Clearmind received FDA Investigational New Drug (IND) approval to begin its Phase I/IIa clinical trial of its MEAI-based CMND-100 capsule for AUD in the U.S. The trial was previously approved in Israel. Clearmind has partnered with leading U.S. institutions, including Yale School of Medicine and Johns Hopkins University School of Medicine, to conduct the study. In Israel, the trial will be conducted at the IMCA Centre in Ramat Gan.
Clearmind’s second focus is MEAI-based food and supplements. The company has entered the non-alcoholic beverage market with its proprietary neurologically active novel alcohol replacement beverage, which has been granted patents in the U.S., India, and Europe. This summer, Clearmind entered into a strategic partnership with JS First to source global manufacturers and distributors for its MEAI-based alcohol substitute beverages, marking a major step toward global expansion.
Mr. Haden says that the innovative MEAI-based beverage, designed as a healthier alternative to alcohol, could be commercially available within two years and is poised to revolutionize the industry by providing the same social benefits as alcohol without the associated negative health impacts.
“MEAI is highly effective with compulsive behaviors because it offers a pleasant experience, and we are very optimistic about its future in the market. I believe MEAI’s ability to treat AUD is going to be profound,” he maintains.
Looking ahead, Dr. Zuloff-Shani says, “We are at the intersection of science and mental health and our vital next steps are to show that MEAI is safe, not addictive, and that it effectively curbs addictive behavior. I am delighted to say that we see initial promising results in all three of these categories.”
• • • • •
To connect with Clearmind Medicine or any other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.