Gritstone bio (NASDAQ:GRTS) is expecting in the first quarter of 2024 preliminary data from its Phase 2/3 study evaluating the Company’s personalized neoantigen vaccine candidate for the treatment of colorectal cancer (CRC).
“The discovery of checkpoint inhibitors was a major development that extended the lives of many cancer patients; however, these treatments are not as effective in patient with cold tumors,” Andrew Allen, M.D., Ph.D. cofounder, president and CEO of Gritstone, says in an interview with BioTuesdays.
Dr. Allen explains that ‘cold’ tumors don’t show evidence of immune activation following treatment with checkpoint inhibitors, indicating that these tumors have effectively hidden themselves from the immune system.
“The neoantigen vaccine field was born when it became apparent that a T-cell response to checkpoint inhibitors is required for a therapeutic benefit,” he says. “Building on the concept of neoantigen vaccines is personalized cancer vaccines developed based on the mutations found in the patient’s tumor. The challenge is identifying which of numerous mutations to incorporate into a vaccine.”
Gritstone developed EDGE, an AI platform designed to identify critical T-cell vaccine targets. EDGE uses deep learning mathematics to build predictive models of which mutated peptides would be displayed on the tumor cell surface for recognition by T-cells.
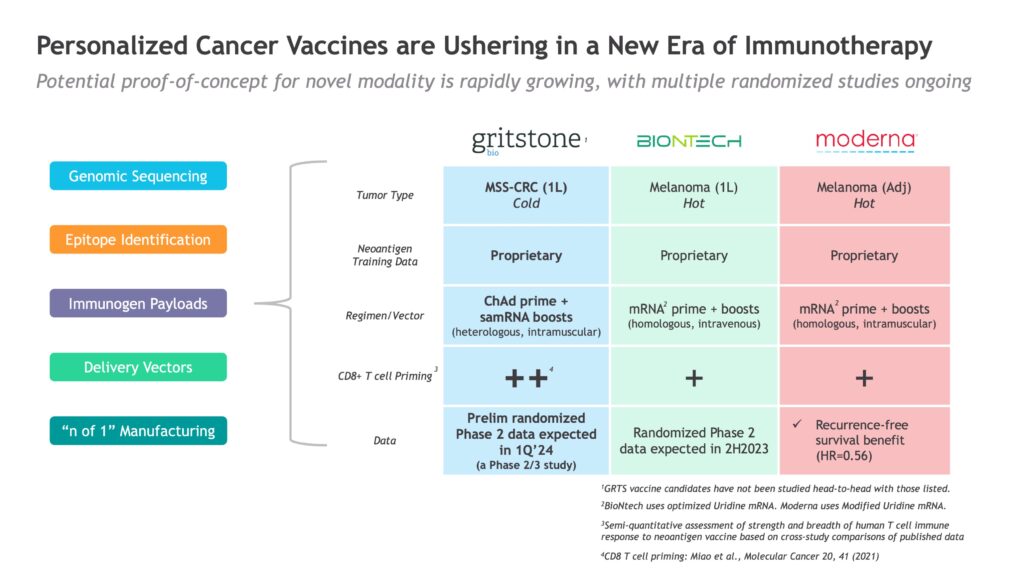
Dr. Allen says that the platform currently has a greater than 80% positive predictive value. “EDGE enables us to identify the top 20 patient-specific neoantigens most likely to generate an immune response in that particular patient,” he says.
Gritstone’s approach to inducing an immune response in cold tumors is priming the immune system with an adenovirus vaccine, followed by a self-amplifying mRNA-based booster. The company has identified microsatellite-stable (MSS) CRC as its proof-of-concept indication, in a program it calls GRANITE.
In a Phase 1/2 trial in patients with advanced solid tumors including MSS-CRC, GRANITE was evaluated in combination with nivolumab and ipilimumab and compared to the combination of nivolumab and ipilimumab alone. GRANITE was well tolerated with no dose-limiting toxicities and demonstrated robust T-cell activation against targeted neoantigens. Six-of-eleven patients had a reduction in their level of circulating tumor DNA (ctDNA), and all subjects that exhibited a reduction in ctDNA had extended overall survival.
“Patients that did not have a molecular response had a median overall survival of about seven months, compared to at least 22 months in patients with molecular responses. The extended median survival in molecular responders suggests that our vaccine is indeed killing those tumor cells,” Dr. Allen contends.
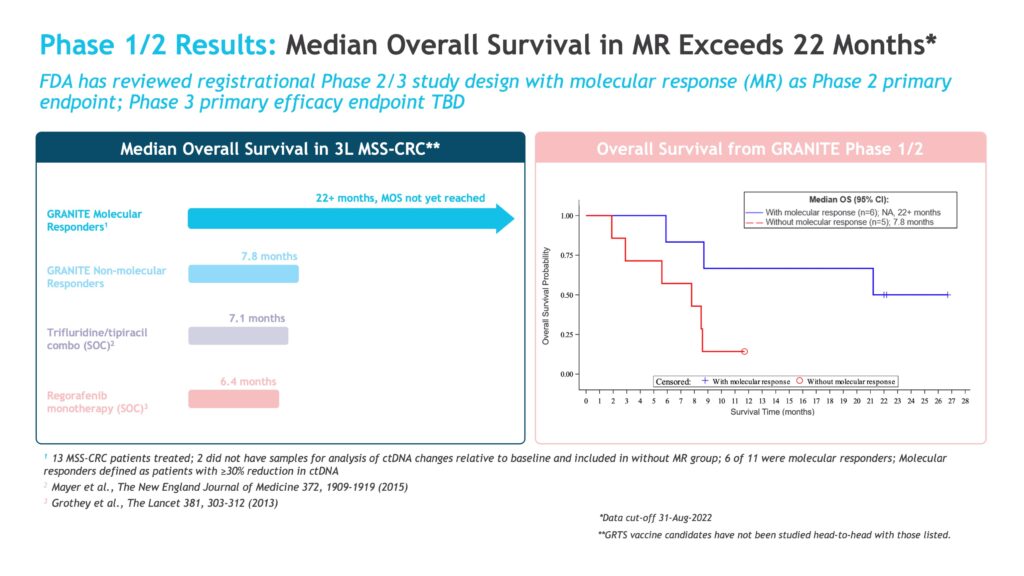
Gritstone’s current Phase 2/3 trial is being conducted in patients with newly diagnosed metastatic CRC, an indication for which GRANITE received FDA fast-track designation in 2018. GRANITE is being evaluated in combination with a checkpoint inhibitor, fluoropryrimidine and bevacizumab with a primary Phase 2 endpoint of a reduction in ctDNA and/or an increase in overall survival. Preliminary Phase 2 data are expected in the first quarter of 2024.
“Positive Phase 2 results would validate our platform, support a Phase 3 trial, and could unlock numerous other cancer indications,” he notes.
The company is using the same antigen selection process and vector delivery systems for ‘off-the-shelf’ neoantigen vaccines, with the notable candidate, called SLATE, targeting KRAS, one of the most commonly mutated genes in solid tumors. In a Phase 1/2 proof-of-concept trial, MSS-CRC and non-small-cell lung cancer patients that were molecular responders demonstrated a median overall survival of 9.6 months, compared to 4.5 months in non-responders.
“In SLATE, we observed a 40% molecular response rate, and in that patient population, ctDNA decreased and overall survival approximately doubled,” Dr. Allen points out. “The patterns look very similar to what we saw with the GRANITE program, and I take great comfort in that, because if a biological signal is real, it’s going to be reproducible.”
Gritstone has since incorporated multiple antigens in a newer version of SLATE intended to enter a Phase 2 clinical trial in 2024.
The company is also applying its platform technologies to infectious disease. “When COVID-19 came along, it was obvious that the spike protein would mutate, so vaccines based on the spike protein would quickly become obsolete. We used our EDGE platform to predict which conserved regions of SARS-CoV-2 were likely to be good T-cell targets,” he explains.
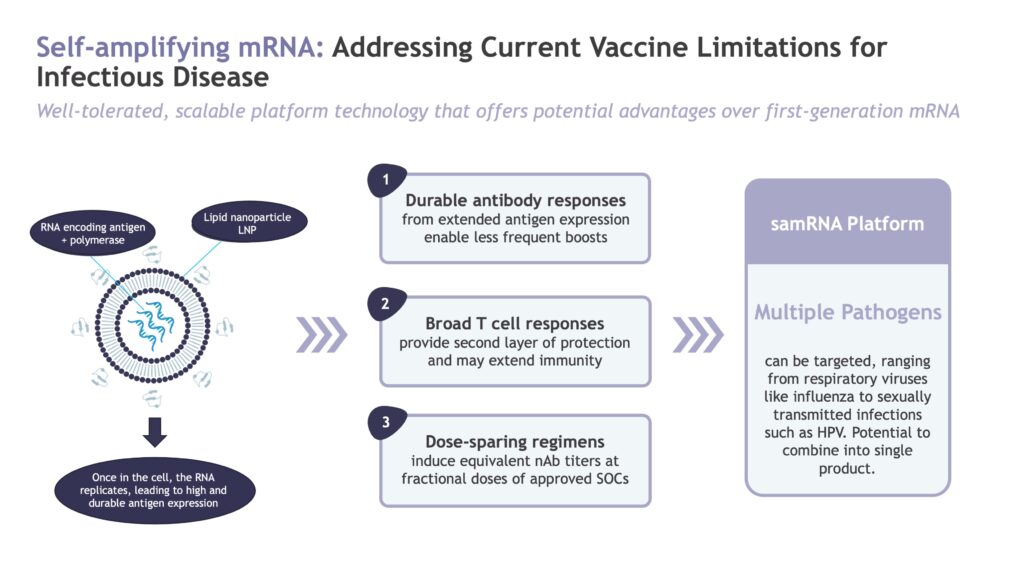
Gritstone’s self-amplifying mRNA COVID-19 vaccine, CORAL, will be evaluated in a 10,000-patient Phase 2b study sponsored by the Biomedical Advanced Research and Development Authority, or BARDA. The head-to-head study is expected to commence in the first quarter of 2024 and will compare CORAL to FDA-approved mRNA-based COVID-19 vaccines.
“Phase 1 CORAL data demonstrated a diverse T-cell response and a durability of at least 12 months, which is superior to currently available COVID-19 vaccines,” Dr. Allen says. “We look forward to the Phase 2b data, and to the possibility of expanding Coral to treat and/or cure other infectious diseases.”
• • • • •
To connect with Gritstone bio or any other companies featured on BioTuesdays, send us an email at [email protected].