Closely-held Intrinsic Medicine is developing new drugs for diseases that are linked to the disordered interaction of the gut microbiome, the immune system and the nervous system.
“The scientific literature makes it clear that the gut-immune-brain axis is central to human health and when dysregulated, it can be an upstream cause of chronic diseases and conditions, yet we don’t really have intentionally designed drugs that can address this dysregulation,” Alex Martinez, CEO, chairman and co-founder of Intrinsic, says in an interview with BioTuesdays.
The gut-immune-brain axis, or GIBA, is a complex system whereby the brain modulates gut function through the hypothalamic-pituitary-adrenal axis and the autonomic nervous system. In turn, gut microbiota modulate the central nervous system via the enteric nervous system, vagus nerve, circulatory system, and immune system.
Diseases and disorders linked to a dysregulated GIBA include autoimmune arthritis, Parkinson’s disease (PD), autism, inflammatory bowel disease (IBD) and even cancer.
Mr. Martinez notes that fecal matter transplants (FMTs) were the first treatments to target the GIBA, but they have significant limitations. “FMTs rely on donor feces and carry infection risk and grafting liability. The second wave of GIBA treatments were live biotherapeutics, including probiotics, but those came with additional challenges of limited consortia and unknowns including the unintentional introduction of antibiotic-resistant genes that could horizontally transfer into the microbiome,” he explains.
The ‘third wave’ focused on postbiotics, which are small-molecule metabolites. “Postbiotics are highly bioactive, at extremely low concentrations, so there are concerns with balancing molecular stability and potential systemic effects,” Mr. Martinez adds.
Intrinsic is taking the prebiotic approach by feeding existing gut bacteria with oligosaccharides to restore the microbiome. The company developed a library of more than 200 lead prebiotic candidates based on human milk oligosaccharides, or HMOs.
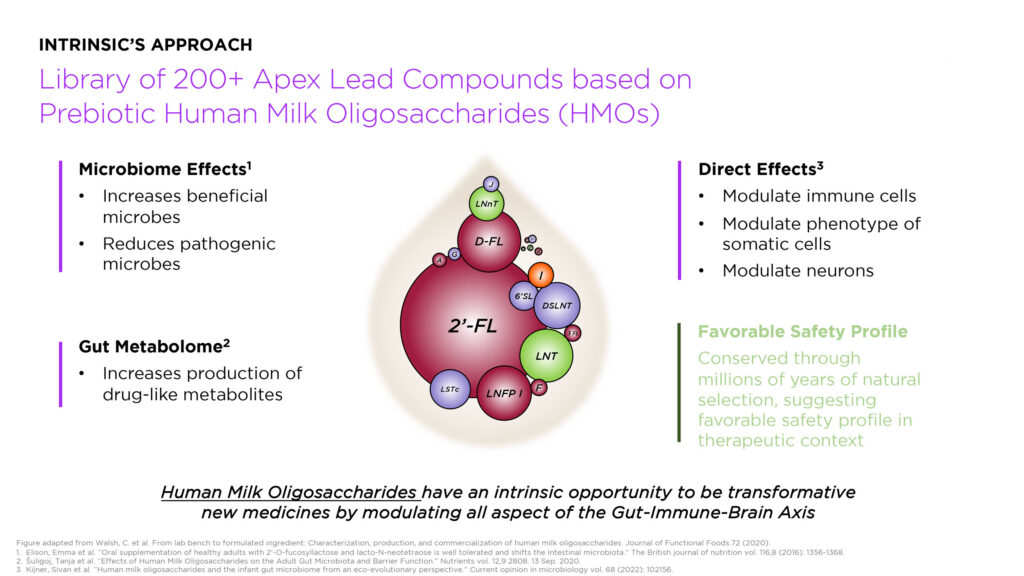
“HMOs have a favorable safety profile and multiple confirmed benefits, including the cultivation of a healthy microbiome; the balancing of innate and adaptive immune responses; and somatic cell maturation, pathogen anti-adhesive, and local and systemic anti-inflammatory effects,” he points out.
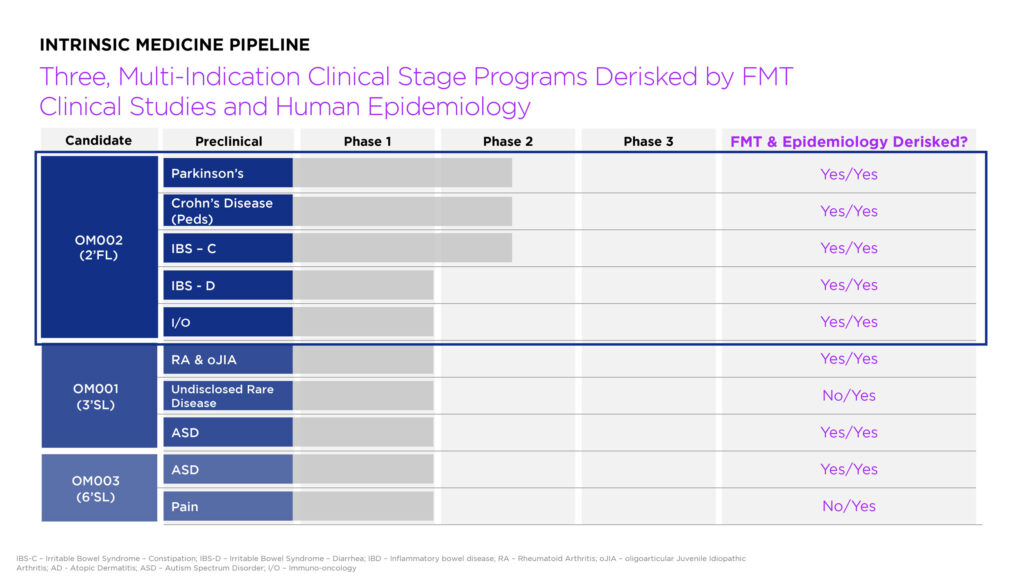
The company’s lead candidate is OM002 (2′-fucosyllactose, or 2’FL), which is the most abundant HMO, constituting nearly 30% of total HMO concentration in most human milk. Intrinsic is developing OM002 for the treatment of PD, Crohn’s disease, irritable bowel syndrome (IBS) and an undisclosed immune-oncology indication.
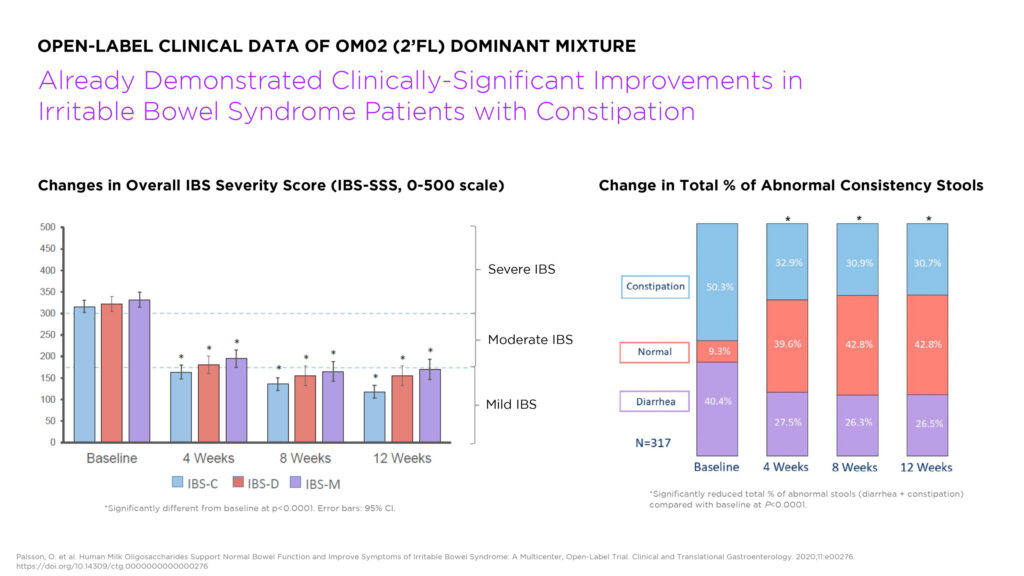
Mr. Martinez says that clinically significant improvements in IBS with constipation (IBS-C) were observed in an open-label study conducted by a manufacturer of a 2’FL-dominant HMO mixture, and points to a controlled Phase 2b study of linaclotide, which is marketed in the U.S. under the brand name Linzess.
“We looked at this study’s placebo responses to gauge if there was a real signal in the open-label study. With the caveat that these were not head-to-head studies, we were encouraged to see that not only was the open-label study’s efficacy in IBS-C superior to the linaclotide placebo response, but that the HMOs appeared to exceed the efficacy of linaclotide on a number of important measures including abdominal pain and bloating. These data indicate that OM002 should be tested further as a new potential IBS drug in properly placebo-controlled clinical studies,” Mr. Martinez says.
Intrinsic received approval this October in Australia to conduct a double-blind, placebo-controlled Phase 2 study to assess the safety and efficacy of OM002 for the treatment of PD with comorbid constipation. Primary study objectives include improvements in the gut and oropharyngeal microbiome profile; and cognition, motor function, and constipation at 12 and 24 weeks.
“What’s striking about PD is that up to 80% of patients have constipation as a preceding symptom,” he points out, adding that a landmark 25,000-patient study published this August in BMJ Gut showed that patients with a history of IBS-C had more than four times the odds of developing the disease.
“There’s a tremendous amount of investment leverage in this study because if we demonstrate an improvement in constipation and any other PD symptoms, we open the opportunity to treat IBS-C and chronic idiopathic constipation as functional risk factors for PD, with expansion opportunities into related ailments including IBD, autism and even long Covid. It a pipeline in a product,” Mr. Martinez adds.
2’FL is also being evaluated for IBD in an ongoing investigator-initiated Phase 1b/2a study taking place at Cincinatti’s Children’s Hospital Medical Center. The trial, called PRIME, is assessing 1g, 5g, or 10g of OM002 compared to a 2g dextrose placebo in 120 pediatric and young adults who are in stable remission and receiving anti-TNF therapy. Primary study outcomes include gastrointestinal symptom rating scale; bifidobacterium abundance; butyrate production; and safety and tolerability. The company plans to initiate its own IBD clinical program, beginning with Crohn’s disease, next year.
“OM002 is a highly rational drug candidate for inflammatory bowel disease. Human epidemiology shows that people with a gene mutation that prevents them from producing a glycoprotein nearly identical to 2’FL in their GI tract have substantially increased risk of Crohn’s disease,” he notes.
Intrinsic’s two additional ‘pipelines in products’ are OM001 (3’-sialyllactose, or 3’-SL) and OM003 (6’-sialyllactose, or 6’-SL). The company is developing OM001 for rare, chronic inflammatory joint diseases affecting children, making OM001 likely eligible for an FDA rare pediatric disease voucher, if approved.
Oligoarticular juvenile idiopathic arthritis (oJIA) affects some 150,000 children in the U.S. and causes the gradual destruction of the bones in the wrists, hands, and feet. oJIA is the largest subtype of juvenile arthritis, and there are currently no approved treatments for the disease.
“oJIA is actually why I started Intrinsic,” he recalls. “It’s viewed as a milder form of juvenile arthritis, but if a child can’t extend their leg – I don’t consider that to be mild. The current treatments are systemic steroids, steroid infections into affected joints, and anti-TNF therapy, all of which can have severe side effects. We’re on the way to developing a safe and effective alternative.”
• • • • •
To connect with Intrinsic Medicine or any other companies featured on BioTuesdays, send us an email at [email protected].