ENDRA Life Sciences (NASDAQ:NDRA) has developed a novel assessment tool – Thermo Acoustic Enhanced Ultrasound (TAEUS) – to enhance existing ultrasound systems and provide capabilities that are available with MRI, but at a cost 50 times lower, and at the point of patient care.
“We are initially focused on using TAEUS to measure fat in the liver as a means to assess and monitor non-alcoholic fatty liver disease (NAFLD), which can progress to NAFLD with inflammation (non-alcoholic steatohepatitis or NASH), chronic liver conditions that affect nearly two billion people globally, and for which there are no practical diagnostic tools,” Francois Michelon, chairman and CEO of ENDRA, says in an interview with BioTuesdays, adding that a liver biopsy is painful, invasive and carries the risk of internal bleeding.
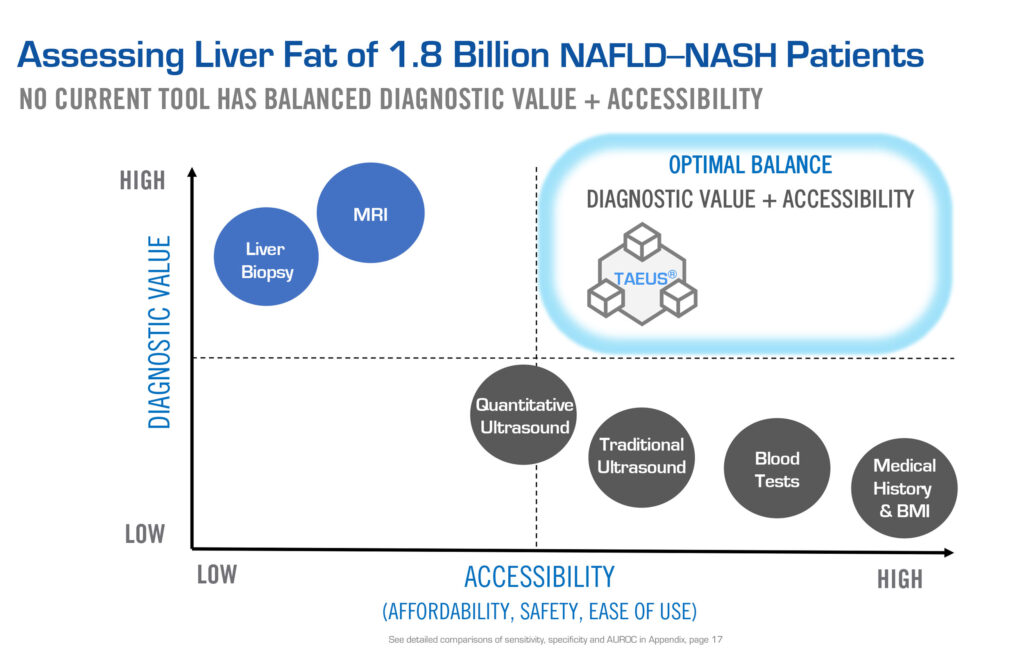
“MRI is the gold standard of the imaging world but not everybody has access to MRI’s. They’re expensive, slow, bolted to the ground and weigh five tons,” he points out. “So, if you want to broaden access to health care, you have to develop something that is high performing, but also accessible, practical and has the economics to make it scalable.”
Mr. Michelon explains that traditional ultrasound simply sends sound waves into tissue, which then picks up an echo coming back out of the tissue.
“TAEUS, which is CE Marked for sale in Europe, is entirely different,” he contends. “It sends radio frequency waves into target tissue that are absorbed, creating small sonic waves that represent a unique signature for lean and fatty tissue. These sonic waves are detected by a separate TAEUS ultrasound receiver, which generates ultrasound images or quantitative data. So, we are radio frequency in, and ultrasound out, a sort of hybrid of MRI and ultrasound, and we see tissue differently than traditional ultrasound.”
ENDRA’s first human feasibility study with 19 subjects compared TAEUS with the gold standard, MRI-proton density fat fraction. “Though it was a small feasibility study, the data were compelling,” Mr. Michelon says.
“Approximately 88% of the time, we were able to tell if liver fat exceeded a 6% normal threshold; 82% of the time we were able to detect liver fat below a 6% threshold, and we tracked very closely to MRI over a range of fat concentrations, with a measurement of approximately 91% using the area under the receiver operating curve metric.”
The FDA is currently reviewing ENDRA’s 510(k) marketing application for TAEUS.
The company hopes to add its TAEUS probe to multiple specialized probes now used with traditional ultrasound machines to enhance clinical workflow and measure fatty liver.
“While we’re aiming to start as a hardware and software accessory, we have the potential for additional revenue streams from services, disposables and licensing,” Mr. Michelon suggests. GE Healthcare, an ultrasound market leader, is currently supporting ENDRA’s introduction to GE customers. “One day, we envision adding our technology into GE and other ultrasound machines, much the way INTEL is a licensed accessory built into computers.”
ENDRA’s TAEUS platform technology is protected by 89 IP assets. “We’re not claiming we’re as good as MRI, but you don’t need to be. We have something that is very good and has other benefits, such as portability, mobility and affordability,” he adds.
Mr. Michelon figures TAEUS will sell for around $50,000, which compares with MRI at a cost of $2.5-million to $3-million per machine.
The annual U.S. medical costs for NAFLD-NASH are estimated at more than $100-billion and fatty liver disease can increase the risks of cardiovascular disease, Type 2 diabetes and chronic kidney disease.
Mr. Michelon says that in addition to measuring tissue composition, TAEUS has opportunities in a variety of other clinical applications, such as measuring tissue temperature change to guide surgery, which can only be done with MRI.
“There are millions of procedures performed annually that depend on either hot or cold ablation technology to treat cancer, to dull pain in a nerve and cardiovascular procedures. Today, there is very little real-time guidance of the tip of an ablation needle, so TAEUS has the potential to show clinicians and surgeons where that energy is going for safer outcomes.”
The company also is open to partnering discussions to license TAEUS for tissue perfusion and vascular flow applications, he adds.
According to Mr. Michelon, the TAEUS liver fat procedure requires less than one day of user training, with each scan taking about 1.5 seconds.
Seven clinical sites currently are evaluating or preparing to evaluate TAEUS against MRI to build additional clinical evidence of TAEUS’ efficacy and safety, refine the technology and for commercialization support. The independent studies include three sites in the U.S. as well as one each in the U.K, Germany, France and Switzerland. Each of the studies, with an average of 75 subjects, is expected to take nine months to complete, “so, we anticipate seeing the first results in 2022,” Mr. Michelon figures.
TAEUS’ liver application is targeting high-value industry segments, such as clinicians, which include radiologists, gastro-hepatologists and endocrinologists, as well as pharmaceutical companies and contract research organizations. “The advent of the first targeted pharma therapies for NAFLD-NASH increases the need to identify and monitor patients.”
Mr. Michelon estimates there are some 50 drugs in development for NAFLD-NASH, of which about 30 are in Phase 2 and 3 programs. “We could see the first therapies in approximately two years.”
In the pharma sector, he says TAEUS has the potential to reduce screening failure rates, which run as high as 55% in NASH studies; measure liver fat more frequently and monitor changes between MRI and biopsies; and support commercialization with front-line clinical identification and monitoring target patients on therapy.
To date, ENDRA has partnered with Hepion Pharmaceuticals (NASDAQ:HEPA) and VGI Health Technology (NSX:VTL) of Australia to pilot the use of TAEUS in their upcoming Phase 2b and Phase 2 NASH programs, respectively, in 2022. Hepion expects to enrol more than 300 liver disease patients in its Phase 2b ASCEND-NASH trial.
ENDRA also is working with the Ladak Laboratory at Western University in London, Canada to develop TAEUS’ artificial intelligence capabilities to improve its algorthyms and automate certain manual measurements.
“Fatty liver disease is just the tip of the iceberg for ENDRA and, even though measuring liver disease is a big story, the business can scale well beyond the liver,” Mr. Michelon says.
• • • • •
To connect with ENDRA Life Sciences or any of the other companies featured on BioTuesdays, send us an email at [email protected].