After close to three decades developing its artificial intelligence platform, Hepion Pharmaceuticals (NASDAQ:HEPA) is deploying its AI-POWR machine learning platform in its clinical program to tackle non-alcoholic steatohepatitis (NASH) and other potential diseases, such as liver cancer.
“We intend to utilize AI-POWR to drive both our ongoing Phase 2a NASH program and identify additional potential indications for our CRV431 drug candidate to expand our footprint in the cyclophilin inhibition therapeutic space,” CEO, Robert Foster, PharmD, Ph.D., says in an interview with BioTuesdays.
“This platform also can help us identify additional therapeutic products to expand our pipeline beyond cyclophilin inhibition,” he adds. Cyclophilins are enzymes that regulate protein folding and their inhibition can be used to treat a variety of diseases.
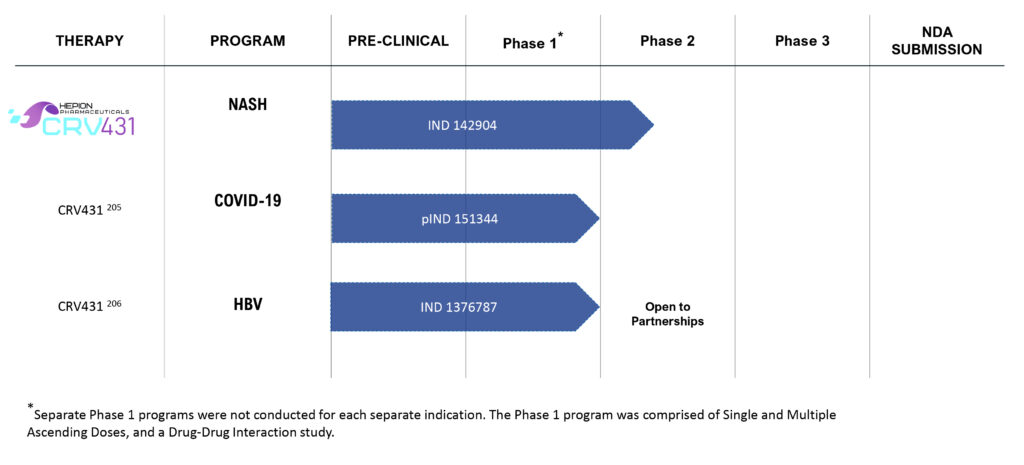
Pipeline
Dr. Foster says he isn’t aware of any other company using AI to optimize development of a treatment for NASH, a potentially life-threatening buildup of fat, inflammation, and scarring of the liver that leads to hepatitis, cirrhosis or liver cancer.
“We believe NASH is a very heterogenous disease with complex biochemical and pathological processes complicated, for example, by time and genetics,” he adds. “So, treating NASH may be tantamount to hitting a moving target.”
Dr. Foster suggests Hepion believes it can mitigate much of the usual risks associated with clinical trials, and NASH in particular, by utilizing cutting edge machine learning tools allowing for precision medicine to further advance CRV431 in the clinic.
AI-POWR is Hepion’s acronym for Artificial Intelligence – Precision Medicine; Omics that include genomics, proteomics, metabolomics, transcriptomics, and lipidomics; World database access; and Response and clinical outcomes.
Dr. Foster explains that AI-POWR consists of data mining access to millions of clinical findings across omics; developing algorithms to fine tune patient selection for clinical trials and enriching study design; and demonstrating improved response outcomes by de-risking failures to save time and money.
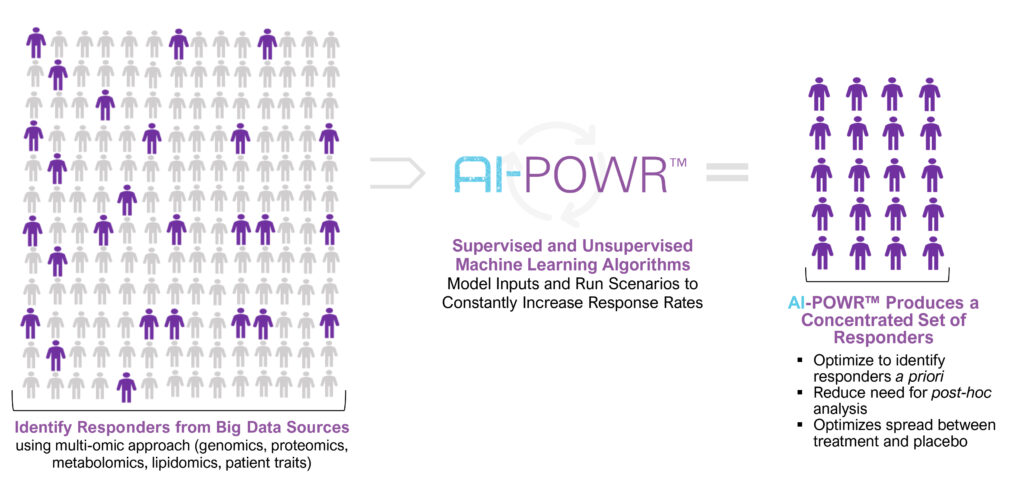
Identifying Responders Increases “Signal-to-Noise”
“AI-driven bioinformatics sets us up better for success,” he contends. “We believe AI-POWR is a risk-mitigation strategy we will employ all the way from clinical trials to commercialization.”
The AI platform is part of a new clinical pharmacology analytics unit and will be led by Patrick Mayo, Ph.D., Hepion’s SVP of clinical pharmacology and analytics. Dr. Mayo receive an award for AI in 1997 for “Applications of Neural Networks,” co-authored by Dr. Brian Corrigan, currently global head of clinical pharmacology at Pfizer.
Hepion expects to complete its Phase 2a AMBITION clinical study with CRV431 in NASH patients no later than the end of the second quarter of 2021, Dr. Foster says, suggesting that a following Phase 2b study could qualify as a registration trial, with a projected NDA filing in 2025.
“We did a ton of preclinical work with CRV431 and we have no doubt that we have a strong anti-fibrotic molecule that has also demonstrated strong safety signals in human trials with single and multiple ascending doses,” he adds. “In each of our eight animal studies conducted by independent laboratories around the world, we showed a reduction in fibrosis.”
For example, Dr. Foster recalls a U.K. study of AI-POWR with CRV431 in human ex-planted livers having fibrosis that analyzed more than 28,000 genes and gene variants. “We identified key NASH-related genes that were significantly and positively altered by only three days of treatment with CRV431.”
According to Dr. Foster, CRV431 is a pan-cyclophilin inhibitor that has a regulatory role in many disease pathways, making them good candidates as drug targets.
Cyclophilins have been shown to play negative roles in viral hepatitis, cancers, acute and chronic lung injury, myocardial infarction, stroke, arthritis, atherosclerosis, thrombosis, aortic aneurysm, coronary artery disease, pulmonary arterial hypertension, ALS, Alzheimer’s disease, multiple sclerosis, muscular dystrophies and traumatic CNS injury.
Pointing to CRV431’s mode of action, Dr. Foster suggests that CRV431 has the potential to bind to cyclophilin A to reduce inflammation; to bind to cyclophilin B to reduce fibrosis by controlling collagen production; and to bind to cyclophilin D to mitigate damage to mitochondrial cells that are the power plants in the body.
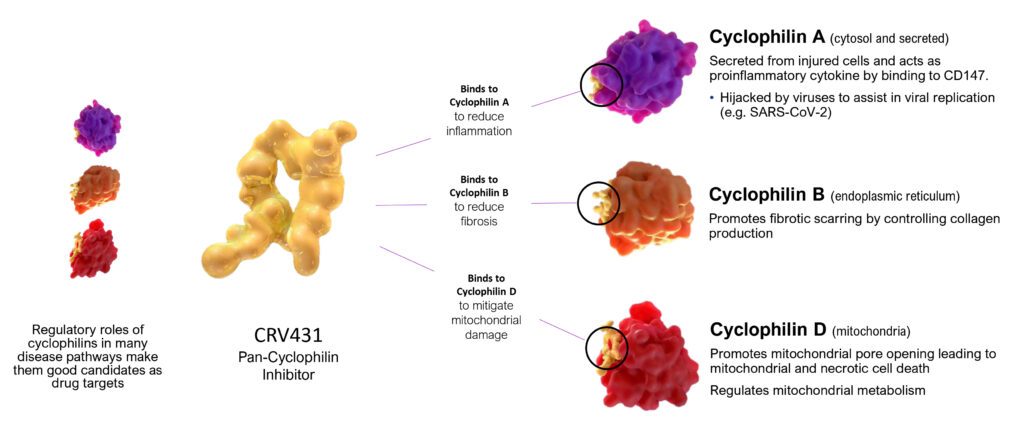
CRV431 MODE OF ACTION
In its ongoing Phase 2a study, Hepion is evaluating 36 stage 2 and stage 3 NASH fibrosis patients divided into two cohorts to receive either low or high oral doses of CRV431, compared with placebo, over 28 days of dosing at 10 clinical sites. There is an observation/follow-up between days 29 and 42.
The study is designed to measure anti-fibrotic activity of CRV431, but more important, Dr. Foster says, will be using AI-POWR to analyze biomarker data, along with multi-omic analyses (e.g., genome, proteome), and patient/disease characteristic to assess overall clinical outcomes and health of the patients. Bioinformatic information gleaned from the Phase 2a study will be used to guide patient selection, trial design, and outcomes for a planned Phase 2b trial to start in mid-2021.
The company expects to complete before the end of 2020 the first cohort of 12 patients receiving 75 mg of CRV431 and six patients receiving placebo. The dose in the second cohort of 12 patients is 225 mg of CRV431.
Hepion also is conducting a long-term toxicology study with CRV431 in monkeys and rats, and a drug-drug interaction study, all to be completed in the second quarter of 2021.
“We’ll continue to refine and extend AI-POWR for NASH and other indications, such as COVID-19 and liver cancer,” Dr. Foster suggests, adding that liver cancer could be Hepion’s next indication.
“We have animal data from a study at Scripps Research Institute that CRV431 had a positive effect on the number and size of liver tumors. The data were compelling and statistically significant.”
• • • • •
To connect with Hepion or any of the other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.