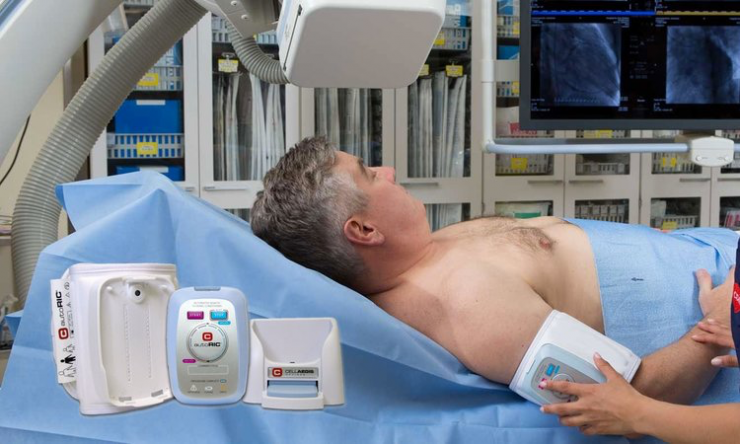
In addition to several large ongoing investigator-sponsored studies in Europe and Canada using its autoRIC device for automated remote ischemic conditioning (RIC), closely-held CellAegis Devices expects its own U.S. pivotal trial for the treatment of elective percutaneous coronary intervention (ePCI) to complete enrolment before the end of 2018.
“RIC has broad clinical evidence dating back to 1986, demonstrating protection in heart, kidney, and brain. This includes heart attacks, elective stenting, stroke and imaging procedures to prevent contrast-induced kidney injury,” CEO, Rocky Ganske, says in an interview with BioTuesdays.
“With our innovative automated device, we are making this protective therapy easily accessible to healthcare providers,” he adds.
According to Mr. Ganske, the benefits of RIC have been reported in more than 7,000 peer-reviewed articles on local ischemic pre-conditioning and more than 650 scientific papers in limb occlusion RIC. In addition to proven protection of the heart and kidneys, RIC can contribute to significant cost savings for healthcare systems, he adds.
The company’s non-invasive autoRIC device has regulatory approval in Europe and Canada, and is currently limited to investigational use in the U.S.
Mr. Ganske explains that autoRIC uses four, five-minute cycles of limb occlusion and reperfusion to protect the heart against ischemia-reperfusion injury and reduce the amount of damage sustained by the heart. The kidneys also receive protection at the same time.
“By inducing a controlled ischemic event and harnessing several of the body’s adaptive responses, which can condition the body against ischemia, tissues are not injured and function is improved,” he adds. “An anti-inflammatory effect also contributes to preventing endothelial dysfunction and platelet activation.”
Mr. Ganske says the autoRIC solution ensures easy, safe, reliable and accurate delivery of RIC with a fully automated, single-button start. The disposable single-use cuff also reduces the risk of infections.
The company’s technology is covered by more than 100 patents worldwide and more than 40 pending patents.
The autoRIC device can be applied in the back of an ambulance by paramedics or in hospitals during a heart attack, “which we call per-conditioning” and for chronic rehabilitation in home care recovery after a cardiovascular event, “which we call chronic-conditioning.”
According to published peer-reviewed studies, RIC can provide significant benefits in patient outcomes. For example, RIC can contribute to a 43% improvement in clinical outcomes of heart attacks, a 42% reduction in kidney damage, a 32% reduction in heart damage from ePCI and a 20% cost savings in heart attack treatment and care in Europe.
Mr. Ganske says recruitment has been completed in two investigator-sponsored trials to further assess the efficacy of RIC in reducing clinical events in patients presenting with ST-segment-elevation myocardial infarction (STEMI). Both trials utilized CellAegis’ autoRIC device to deliver RIC therapy and are designed to support expanded adoption of CellAegis’ technology in Europe.
Both trials are investigating the difference between RIC and standard-of-care treatment in reducing cardiac death and hospitalization for heart failure in STEMI patients.
One study, CONDI 2, sponsored by Aarhus University Hospital in Denmark has enrolled 2,600 STEMI patients in ambulances in Denmark, Serbia, and Spain. The second study, ERIC-PPCI, has enrolled 2,700 STEMI patients across 20 National Health Service hospitals in the UK. It was sponsored by the London School of Hygiene and Tropical Medicine. Trial results from both studies are expected in the second half of 2019.
Last month, CellAegis entered into a definitive agreement with Cardiologic to market and distribute autoRIC in the UK and Ireland. “This marks our first distribution agreement for autoRIC outside of Canada and is a significant step forward for our international commercialization strategy,” Mr. Ganske says. The company is currently considering a distribution partner for Germany.
In addition, the William Osler Health System in Ontario is currently sponsoring the 1,800-patient FIRST field implementation study, with enrollment scheduled for completion in August. Patients experiencing a heart attack are treated with the autoRIC device either in the ambulance by Peel or Halton emergency medical services or in the emergency department or catherization lab of Brampton Civic Hospital or Trillium Healthcare Partners.
The benefit of the autoRIC device treatment will be assessed by comparing clinical outcomes, including hospital readmissions and future heart attacks, of treated patients with those of untreated heart attack patients. “Following the completion of this study, we anticipate that autoRIC treatment will be added to standard treatment for heart attacks in the Region of Halton-Peel, followed by wider Ontario and Canadian adoption,” Mr. Ganske adds.
In January, CellAegis enrolled the first patients in its SHIELD pivotal trial in the U.S., a 500-patient single-blind clinical trial designed to evaluate the safety and effectiveness of the autoRIC device to attenuate myocardial injury, as measured by a validated cardiac biomarker, troponin 1, in patients undergoing ePCI.
Patients scheduled either for an ePCI procedure, or a diagnostic catheterization procedure that may be followed with stenting, will be enrolled in the study across 15 research sites in the U.S. and Canada. The company expects to complete enrollment in the third quarter of 2018.
The primary efficacy outcome of the U.S. trial is to show a statistically significant reduction in the prevalence of ischemia-reperfusion injury to the myocardium in patients using the autoRIC device, compared with patients using a placebo device. Patients will exit the study after completion of their 30-day post-procedure follow-up. The company hopes to file for FDA approval by the end of 2018.
“There are approximately one million ePCI procedures performed annually in the U.S. in patients with ischemic heart disease, many of whom have procedural related troponin elevations, which have been shown to result in poorer clinical outcomes,” Mr. Ganske points out.
“We are in a unique position of having our device used by investigators in multiple cardiovascular studies, where we are paid for the use of the device and consumables, and also obtain access to study data for regulatory purposes,” he adds. “This leverages more than $25-million of third-party capital.”
He estimates that the autoRIC cuff market potential in North America and Europe, covering STEMI, ePCI and angiography to prevent contrast-induced kidney injury, is approximately $400-million at launch. Additional future indications, such as stroke and heart failure, would expand the market significantly above $5-billion annually.
• • • • •
To connect with CellAegis, or any of the other companies featured on BioTuesdays, send us an email at [email protected].