Closely-held Tear Film Innovations plans to formally launch at two upcoming medical conferences its iLux medical device to treat Meibomian gland dysfunction, the leading cause of dry eye disease.
The company will be exhibiting to ophthalmologists at the American Society of Cataract and Refractive Surgery (ASCRS) this weekend and optometrists at Vision Source: The Exchange 2018 during the first weekend in May.
“By ASCRS, we’ll have initiated the integration of iLux at 25 eye practices, where they’ll have the proper tools and processes in place to identify dry eye patients, discuss iLux with patients and perform procedures,” CEO, Rob Thornhill, says in an interview with BioTuesdays.
“We designed iLux so patients will say yes to a dry eye treatment without a significant financial commitment and provide doctors with a business model that would benefit their practice,” he adds.
According to Mr. Thornhill, the root cause of dry eye is Meibomian Gland Dysfunction (MGD), which is a blockage or some other abnormality of the meibomian glands, causing them to not secrete enough oil to cover the tear film. This oil (lipid) layer is the key to a healthy ocular surface and 86% of dry eye patients have an insufficient lipid layer.
“Any blockage of meibomian glands can lead to evaporative dry eye, resulting in unstable tear film and associated symptoms,” he adds. “iLux is designed to treat the leading cause of the disease.”
Tear Film Innovations was founded in 2014. The technology, resulting in a handheld device designed to unclog meibomian glands with heat and compression, was invented and developed in-house by Brian Kelleher, a co-founder and currently COO of the company, and Kabir Gambhir, another co-founder.
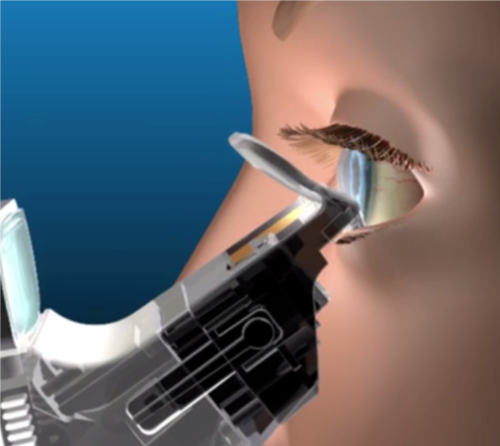
Mr. Thornhill explains that a clinician can view the meibomian gland orifices through a magnifier on the iLux instrument and assess the impact of the heat and compression from the iLux system on the glands. The compression force and heat is controlled by a thumb-operated switch that gives a clinician tactile feedback.
After the meibum is melted, the clinician gently massages the eyelid between the two pads, expressing the melted meibum through the gland orifices, which are monitored through the magnifier.
Unlike other treatments for MGD, the iLux device is designed to accomplish this procedure usually in less than a minute per treatment zone for most patients.
Mr. Thornhill points out that safety features incorporated in the device ensure that the energy delivered is within the therapeutic range, and that the compression force is well tolerated by patients.
While the treatment relieves dry eye symptoms, it is not a cure and he says most doctors want to see patients every six months on average. iLux may improve cataract and LASIK surgical outcomes and complement dry eye medications, including Restasis and Xiidra.
Dry eye disease affects more than 20 million people in the U.S. and 300 million worldwide, with the incidence growing above 5% a year because of an aging population, use of electronic devices, diabetes, contact lens wear and cataract and LASIK surgery.
“Because people blink less while using computers, tablets and smartphones, for example, meibomian glands are not activated to secrete enough oil onto the tear film,” Mr. Thornhill suggests.
Common symptoms of dry eye include dryness, grittiness, soreness, irritation and burning, watering, sensitivity to light, blurred vision and eye fatigue. These symptoms, which often worsen throughout the day, can significantly diminish quality of life. The symptoms of dry eye disease are one of the most common reasons people visit the eye doctor.
Mr. Thornhill says patients in a randomized clinical study had similar outcomes with the iLux and the TearScience LipiFlow. However, the price paid by patients for iLux handheld treatments will be roughly one-third of the price paid by patients for LipiFlow.
iLux also differentiates itself from LipiFlow by allowing doctors to offer customized treatments to patients and to verify outcomes at the time of treatment, he adds.
For clinics, Mr. Thornhill says the iLux instrument is one-third the cost and the disposable one-fourth the cost of the LipiFlow instrument and disposables.
iLux recently received 510(k) clearance from the FDA after completing a pivotal study, including LipiFlow, with 142 patients at eight clinical sites in the U.S. last August.
The study demonstrated that iLux’s meibomian gland score, and measurements of tear breakup time and ocular surface disease index all exceeded the FDA’s requirement for clinical significance. iLux also was found to be non-inferior to LipiFlow. There were no adverse events and iLux was proved to be safe for patients.
“We have a seasoned management team, with a successful track record, and an opportunity to build a cash flow positive company by the end of 2019,” Mr. Thornhill suggests.
• • • • •
To connect with Tear Film, or any of the other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.