Closely held Longhorn Vaccines and Diagnostics is developing peptide vaccines and monoclonal antibodies aimed at preventing and reducing the global burden of medical conditions caused by key bacterial toxins, including both pandemic and endemic diseases in humans and animals.
“We are advancing a robust pipeline of broad based solutions to better diagnose, prevent, and treat a wide range of viral and bacterial infections, as well as the resulting inflammation that underpins numerous debilitating diseases such as Alzheimer’s, Parkinson’s, autism, depression, anxiety, atherosclerosis, select cancers, tuberculosis—and the obvious one, bacterial sepsis,” Jeff Fischer, co-founder and president of Longhorn says in an interview with BioTuesdays.
“Our mission is to develop and deliver accessible therapies that improve health, extend lifespan and quality of life, and address global public health challenges using technologies with long-term safety profiles,” Mr. Fischer says.
Mr. Fischer explains that monoclonal antibodies offer a promising approach for targeting specific pathogens and neutralizing toxins. Accordingly, Longhorn is developing an antibody cocktail against multiple bacterial antigens that may provide broad-spectrum protection against sepsis and other inflammatory diseases. This strategy builds on the more than four decades of experience Longhorn’s management team has in developing antibody-based treatments for bacterial infections.
“Whether used alone or in combination therapies, monoclonal antibodies attack the root cause of chronic inflammation while leaving the immune system fully intact,” he adds.
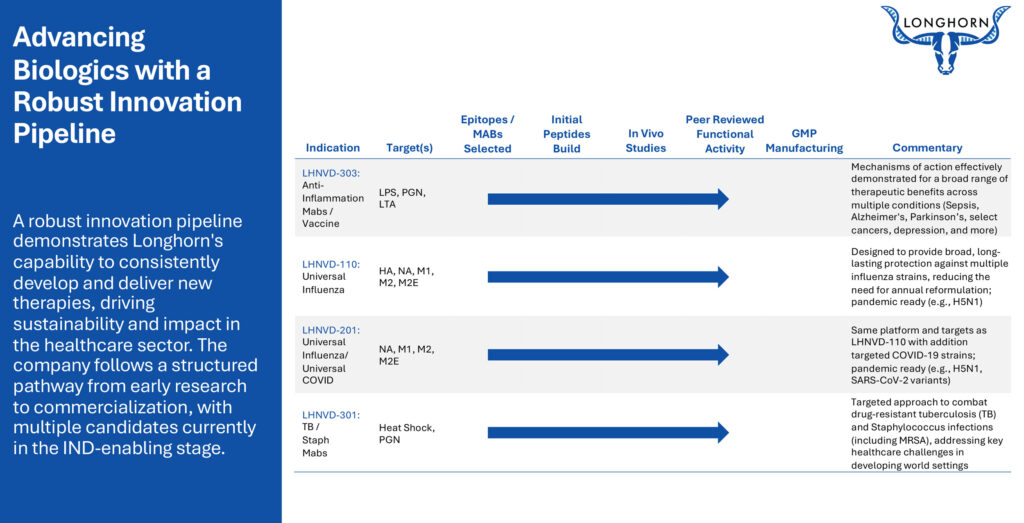
Longhorn’s lead candidate, LHNVD-303, is a broad-based vaccine and monoclonal antibody combination built on a composite peptide platform. It is designed to prevent bacterial infections and clear associated toxins by targeting lipoteichoic acid (LTA), lipopolysaccharide (LPS), and peptidoglycan (PGN) epitopes—three key bacterial cell wall components implicated in sepsis and inflammatory disease.
“We’re really focused on bacterial infections, inflammation, and vaccine development. Our research has shown that the bacterial cell wall is key to attacking these pathogens,” Mr. Fischer says.
LTA, a crucial component of gram-positive bacterial cell walls, activates immune responses through Toll-Like Receptor 2 (TLR2), contributing to septic shock and severe complications like arthritis and multi-organ failure. LPS, found in gram-negative bacteria, triggers strong immune responses via Toll-Like Receptor 4 (TLR4) and lipid A, while also enhancing bacterial resistance to antibiotics—excessive activation can lead to septic shock. PGN is the main structural element of bacterial and mycobacterial cell walls. PGN engages TLR2 and NOD-like receptors to trigger immune responses and helps pathogens like S. aureus evade immune detection.
“Our monoclonal antibody cocktail targeting LTA, PGN, and LPS offers a comprehensive and novel approach to preventing and treating sepsis and inflammatory diseases. By neutralizing key toxins and enhancing bacterial clearance, it has the potential to significantly improve patient outcomes and reduce long-term morbidity and mortality. One major advantage of extended half-life monoclonal antibodies is their great track record for being safe,” Mr. Fischer asserts.
LHNVD-303 targets conserved bacterial components found across gram-positive (e.g., S. aureus, Enterococci, S. pneumoniae) and gram-negative (e.g., E. coli, Pseudomonas) species. It engages the immune system to enhance opsonophagocytosis and clears bacterial cell wall components released during infection.
“Since bacterial cell wall components have been studied and tested in clinical trials for decades, we know that bacteria and their toxins cause inflammation and are central to a wide range of chronic and fatal conditions,” he adds.
Mr. Fischer notes that the adjuvanted composite peptide vaccine—incorporating LTA, LPS, PGN, and tetanus toxoid epitopes—is designed to elicit a broad immune response against both gram-positive and gram-negative bacteria and their toxins. The resulting antibodies can also be developed into monoclonal antibodies for passive immunization. To enhance effectiveness, Longhorn has extended their half-life to last for four to six months, making them suitable for patients unable to mount an immune response.
“Passive immunization enables faster, more controlled evaluation than traditional vaccines, which typically require longer timelines and larger clinical cohorts. Notably, monoclonal antibodies are fully cleared from the body within months, reducing long-term side effect concerns—making them a valuable step toward broader vaccine development and adoption,” he adds.
Mr. Fischer emphasizes that bacterial infections threaten millions worldwide and are further complicated by rising antibiotic resistance. This has led to suboptimal treatment and persistent toxin release. “Adopting active vaccination and passive immunization using extended half-life monoclonal antibodies as standard of care could save millions of lives and dramatically reduce healthcare costs.”
In addition, Longhorn is developing LHNVD-301, a PGN-targeting antibody for the treatment and prevention of tuberculosis in developing regions.
Mr. Fischer adds that the company is also working on LHNVD-110, a universal influenza vaccine, and LHNVD-201, a combined universal influenza and COVID-19 vaccine. Both follow Longhorn’s composite peptide platform paired with extended half-life monoclonal antibodies. “What makes us really excited is that we’re able to target all stages of the viral cycle—binding, replication, and release—offering cross-protective immunity and leaving the virus with no safe haven.”
“Innovation in inflammation therapeutics could rapidly address an unmet $25 billion market,” Mr. Fischer concludes. “Toxin-associated inflammation opens the door to tackling adjacent disease areas with enormous global implications. We believe the Longhorn platform has the potential to transform lives in both the U.S. and worldwide.”
• • • • •
To connect with Longhorn Vaccines and Diagnostics or any other companies featured on BioTuesdays, send us an email at [email protected].