Terns Pharmaceuticals (NASDAQ:TERN) is developing a portfolio of novel, oral small-molecule product candidates based on clinically validated mechanisms of action to address serious diseases including oncology and obesity.
“Our overall impact on human health is deeply important to us. We believe our molecules have the potential to enhance the lives of millions of people affected by serious, life-threatening diseases, including those personally and professionally close to us,” Amy Burroughs, CEO of Terns says in an interview with BioTuesdays.
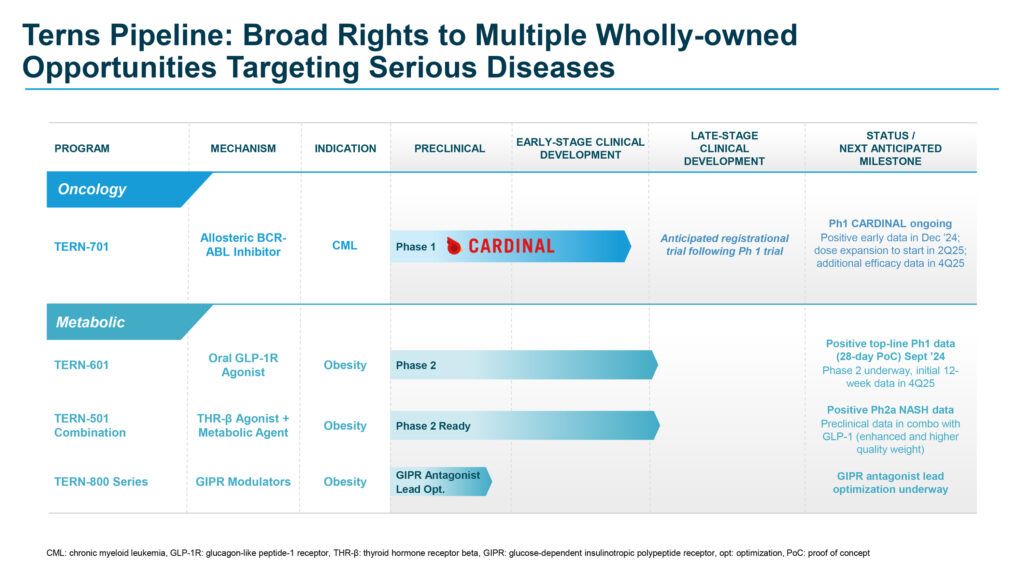
Ms. Burroughs explains that Terns is primarily focused on two proprietary, clinically validated molecules: TERN-701, a potential treatment for chronic myeloid leukemia (CML), and TERN-601, a potential treatment for obesity. In addition, the company’s pipeline also includes a THR-β agonist and a preclinical GIPR modulator discovery effort for obesity.
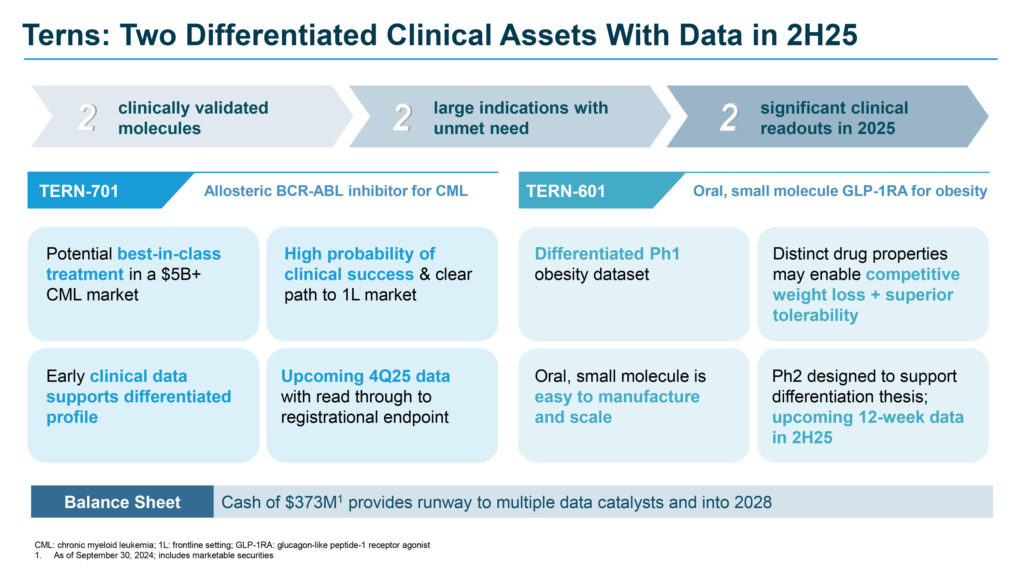
TERN-701, the company’s oral, potent allosteric BCR-ABL tyrosine kinase inhibitor (TKI), has demonstrated superior efficacy and safety compared to traditional active-site TKIs. Designed to target the ABL myristoyl pocket, it is being developed as a potential best-in-class treatment for CML, a life-threatening cancer that originates in the bone marrow. In March 2024, the FDA granted TERN-701 orphan drug designation for the treatment of CML.
In the U.S., CML is considered an orphan indication with more than 9,000 new cases diagnosed annually. Ms. Burroughs highlights that since the introduction of TKI therapy in 2001, CML has shifted from a life-threatening disease to a manageable chronic condition for most patients. However, despite advancements in active-site targeting TKIs, many patients fail to achieve long-term disease control due to resistance or intolerance. This often forces patients to cycle through earlier generation treatments. As a result, both physicians and patients are seeking improved therapies. The allosteric class of TKIs represents a novel class of therapy, with potential for improved efficacy, safety, and quality of life for patients.
“CML is a chronic, orphan indication requiring multiple therapies due to lifelong treatment needs and frequent therapy switches. Allosteric BCR-ABL TKIs, like TERN-701, are the only drug class to demonstrate superior efficacy and tolerability compared to active-site TKIs, marking a significant advancement in CML treatment,” she asserts.
In October 2023, Terns initiated the Phase 1 CARDINAL trial of TERN-701 in the U.S. CARDINAL is a global, multi-center, open-label, two-part Phase 1 clinical trial evaluating the safety, pharmacokinetics (PK), and efficacy of TERN-701 in patients with relapsed or refractory CML.
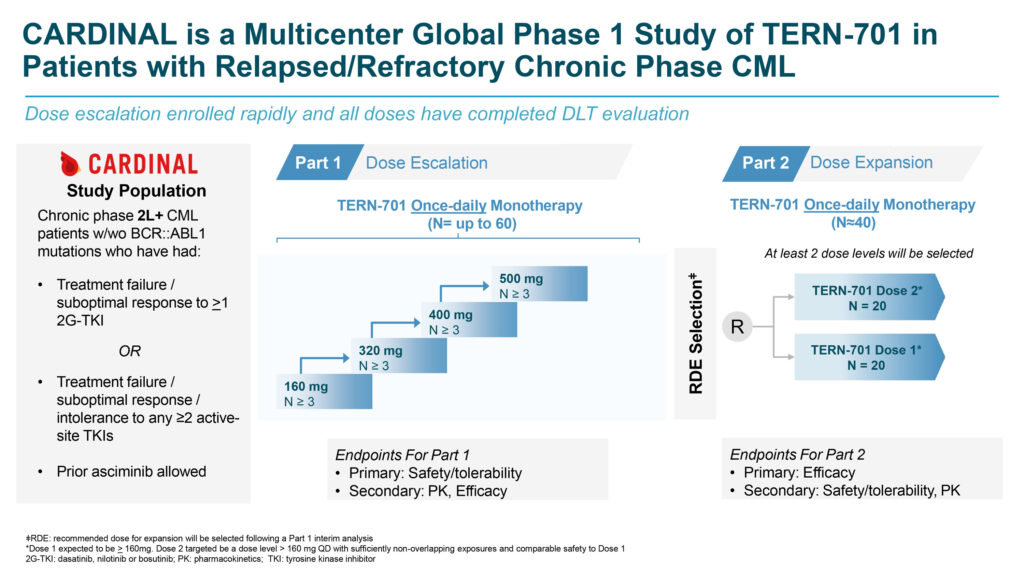
Additionally, an ongoing Phase 1 study of TERN-701 in CML is being conducted in China by Terns’ partner, Hansoh Pharma.
Initial results from the U.S.-based CARDINAL trial, released in April 2024, demonstrated that TERN-701 can be administered once daily, with or without food, at doses that achieve clinically efficacious exposures.
“The ability to dose with or without food is a key potential differentiator from asciminib—the current-standard of care and the only approved allosteric BCR-ABL inhibitor—which requires twice-daily dosing and three hours of fasting with each dose, creating a significant burden for patients,” Ms. Burroughs notes.
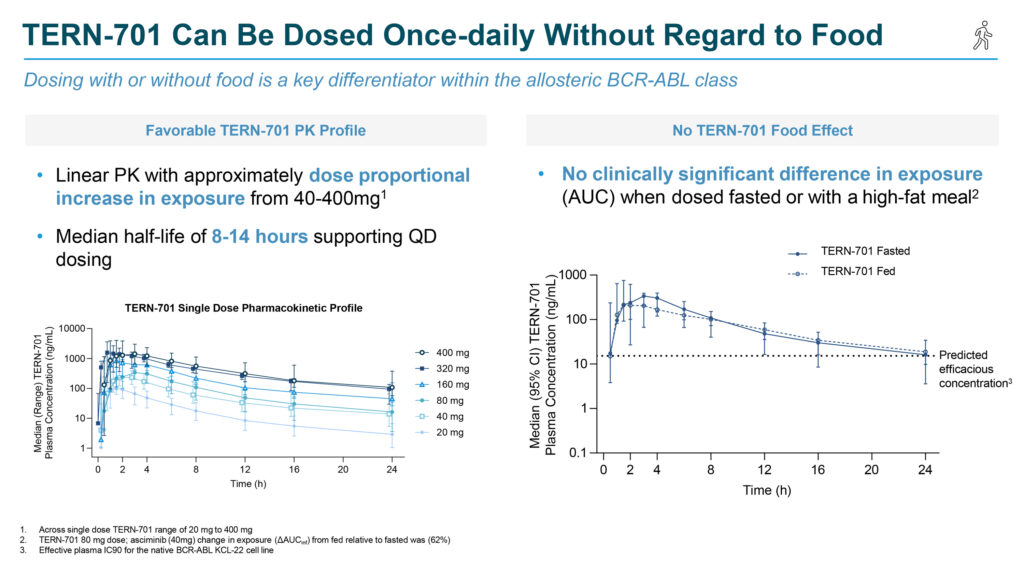
In December, Terns announced additional positive data showing strong molecular responses starting at the lowest dose level in heavily pretreated patients with high baseline BCR-ABL transcript levels. The safety profile remained encouraging, with no dose limiting toxicities, adverse event-related treatment discontinuations, or dose reductions across three dose-escalation cohorts. High levels of target coverage were achieved with once daily dosing at all levels.
“We have a remarkable safety and convenience profile, and we’re conducting a healthy volunteer study that further supports an improved drug-drug interaction profile compared to asciminib. This is critical for safety and convenience because physicians and patients wouldn’t need to adjust other medications—particularly statins, which so many people take—to accommodate TERN-701,” Ms. Burroughs clarifies.
The company completed dose escalation in January 2025 and anticipates initiating dose expansion in the second quarter of 2025, with additional safety and efficacy data, including longer-term major molecular response rates, expected in the fourth quarter of 2025.
Looking ahead, Ms. Burroughs emphasizes that Terns is in a strong financial position to support upcoming milestones. “We are well capitalized with runway into 2028, enabling us to execute on trials, readout these data, and generate additional clinical results.”
“We believe TERN-701 has best-in-class potential in a large and growing CML market. Our early clinical data underscore a differentiated profile with potential efficacy and safety advantages over asciminib, along with more convenient dosing that could broaden treatment options and improve quality of life for people with CML.”
Ms. Burroughs adds, “It’s rare to develop a Phase 1 candidate with such high clinical promise and therapeutic potential for people with CML. TERN-701 has a well-defined path to market and we’re incredibly excited about its potential impact on patients.”
• • • • •
To connect with Terns Pharmaceuticals or any other companies featured on BioTuesdays, send us an email at [email protected].