By Melane Sampson

Arch Biopartners (TXSV:ARCH; QTCQB:ACHFF) is at the forefront of developing breakthrough therapies to combat acute kidney injury (AKI) and prevent organ damage. By targeting the dipeptidase-1 (DPEP1) inflammation pathway—an overlooked yet critical driver of kidney and multi-organ inflammation—Arch is advancing a platform of novel drugs with the potential to transform treatment for millions of patients worldwide.
“The kidney story is very exciting—we are pioneering a novel drug based on a groundbreaking science discovery, offering a potential treatment where none currently exists for patients at risk of kidney failure,” Richard Muruve, CEO of Arch says in an interview with BioTuesdays.
The company is focused on two drug candidates: LSALT peptide and a repurposed drug, cilastatin. LSALT peptide is a non-enzymatic DPEP1 inhibitor designed to target inflammation-induced AKI. Cilastatin, originally developed in the 1980s for preventing an antibiotic from degrading due to DEPE1 in the kidney, is being repurposed by Arch under new method-of-use patents as a standalone treatment for AKI.
AKI affects approximately 15 million people globally each year. With no approved drug treatments available, patients with AKI often require dialysis to survive.
“AKI is often caused by either inflammation or toxins,” Mr. Muruve explains. “Inflammation-related AKI commonly occurs in patients following on-pump cardiac surgery or those experiencing septic shock with multi-organ inflammation. Toxin-induced AKI is a frequent side effect of certain antibiotics, chemotherapeutic agents, or antivirals.”
“While white blood cells play a crucial role in healing injured areas of the body,” he points out, “organs are highly sensitive to the inflammatory process. Organ injury occurs when there are too many white blood cells, causing harmful inflammation.”
Arch’s scientific discovery identified a common inflammatory mechanism of action that occurs in the kidney, liver, and lungs, leading the Arch team to develop LSALT peptide—potentially the world’s first DPEP1 inhibitor to prevent inflammation related to AKI, Mr. Muruve asserts.
The company is advancing LSALT peptide toward regulatory approval, based on the human trial results demonstrating its ability to specifically block DPEP1-mediated inflammation injury to the kidney.
In preclinical AKI models, LSALT peptide successfully targeted the DPEP1 pathway, preventing ischemia-reperfusion injury (IRI) in the kidneys. In August 2019, Arch scientists Dr. Donna Senger and Dr. Stephen Robbins published a peer-reviewed study in the top-tier journal Cell, describing a novel mechanism of action for acute inflammation in the lungs, kidneys, and liver.
The publication identified DPEP1 as a major neutrophil adhesion receptor on the endothelium of the lungs, liver, and kidneys. LSALT peptide was shown to target DPEP1, offering a new approach to treating neutrophil-driven inflammatory diseases. Unlike conventional anti-inflammatory drugs, LSALT peptide focuses on this novel adhesion receptor rather than individual cytokines.
Alongside the study, Arch submitted a video data demonstrating the effects of LSALT peptide in a mouse kidney injury model using intravital microscopy. The footage illustrates what a healthy kidney looks like, a kidney with inflammation caused by IRI induced through oxygen deprivation, and a healthy kidney following IRI and post-treatment with LSALT peptide.
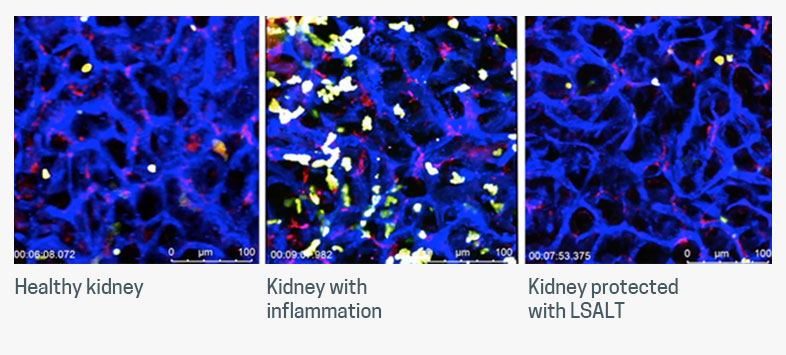
“So, if we achieve this in humans—which is exactly what we’re testing in our Phase 2 trial—we have the potential to transform global kidney care,” he contends. “Reducing the number of patients requiring dialysis or kidney transplants would be a game-changer for patients at risk for this kind of AKI.”
Additionally, in 2024, Arch scientists published a study in the journal BMJ Open describing proof-of-concept results from the company’s 2021-2022 Phase 2 acute lung inflammation trial. “The data from the trial provided the first-ever evidence validating DPEP1 as a mediator of organ inflammation and a viable therapeutic target in humans. Moreover, the article confirmed that LSALT peptide was well tolerated, with no safety issues related to the drug,” Mr. Muruve says.
Arch is now conducting a Phase 2 trial for LSALT peptide targeting cardiac surgery-associated acute kidney injury (CS-AKI), which is caused by ischemia-reperfusion. Patient dosing began in March 2024 across five active hospital sites in Turkey. In late 2024, the University of Calgary began recruiting patients, while the company is prepared to activate two additional sites in Toronto in early 2025. The trial aims to enroll up to 240 patients across Turkey, Canada, and the U.S.
Each year, more than one million cardiac surgeries—including bypass procedures—are performed, with approximately 30% of patients developing AKI. CS-AKI is often caused by IRI, which reduces blood flow (ischemia) and oxygen to the kidneys, causing kidney cell damage. When blood flow is restored (reperfusion), inflammation is triggered, exacerbating injury to the kidneys. Currently, no therapeutic treatment is available to prevent AKI of the type commonly experienced by on-pump cardiac surgery patients. In severe cases of AKI, kidney failure occurs, requiring dialysis for survival.
Arch is also supporting clinical research on cilastatin, a drug with off-target effects that could potentially prevent toxin uptake in the kidneys. “We are partnering with clinical researchers in Canada for a Phase 2 trial investigating cilastatin for toxin-related AKI,” Mr. Muruve notes.
The investigator-led trial titled Prevention of Nephrotoxin-Induced Acute Kidney Injury (PONTiAK), will evaluate cilastatin’s ability to prevent AKI caused by nephrotoxic drugs, including antibiotics, chemotherapy agents, and radiographic contrast. The 700-patient Phase 2 study will rely on Arch to supply the cilastatin drug product.
The PONTiAK trial builds on research published in The Journal of Clinical Investigation (JCI) in 2018, where Arch scientists demonstrated cilastatin’s ability to inhibit leukocyte recruitment and drug-toxin uptake in the kidneys, thereby preventing AKI.
The trial, led by investigators at the Universities of Calgary and Alberta, has received $1.5 million in funding from the Canadian Institutes of Health and an additional $400,000 from the Accelerating Clinical Trials program to support randomized controlled trials in Canada. The study will be conducted at up to five hospital sites in Alberta.
Arch anticipates releasing data from both the LSALT peptide and PONTiAK trials before spring 2026.
Highlighting a key advantage in the company’s regulatory pathway, Mr. Muruve points out that Arch is competing against a disease rather than existing drug therapies. “Reduction of disease and injury prevention is a clear benchmark and provides Arch a clear pathway toward approval, which is possibly much shorter, given the urgent unmet medical need in this area.”
In conclusion, he remarks, “We believe LSALT peptide and cilastatin have the potential to be major breakthroughs in preventing and treating common injuries to the kidneys caused by inflammation and/or toxins that affect millions of patients globally each year.”
• • • • •
To connect with Arch Biopartners or any other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.