By Melane Sampson

Closely-held VICO Therapeutics is developing antisense oligonucleotide (ASO) RNA modulating therapies for patients with severe genetic neurological diseases, including Huntington’s disease (HD) and spinocerebellar ataxia types 1 (SCA1) and 3 (SCA3), by targeting the root cause—cytosine-adenine-guanine (CAG) trinucleotide repeat expansions.
“VICO is a leader in the creation of designer ASOs for multiple genetic neurological disorders, leveraging our proprietary VICOMER platform technology, which we have built upon from our extensive experience in designing, manufacturing, and developing ASOs as RNA-modulating therapeutics,” Micah Mackison, chief executive officer at VICO, says in an interview with BioTuesdays.
“Our ASOs are simple synthetic yet biodegradable stretches of nucleotides that allow high sequence specificity, straightforward optimization of structure-activity relationships, and fine-tuned implementation of chemical modifications. We select a specific genetic type of RNA modulator best suited to correct the genetic defect and apply precision chemistry to achieve the desired efficacy and safety profile,” he adds.
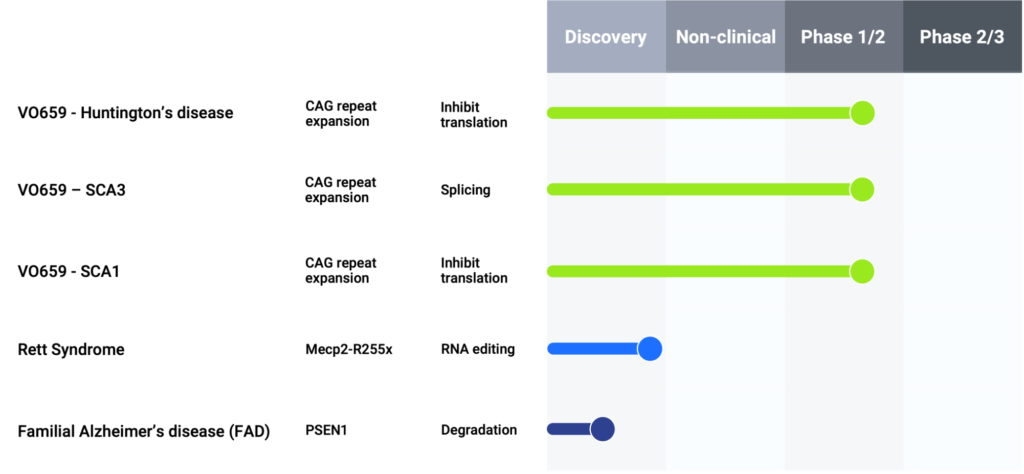
The company’s lead product candidate, VO659, which has FDA and EMA orphan drug designation for the treatment of HD and SCA, is currently in Phase 1/2a clinical development for HD, SCA1, and SCA3. VO659 is an investigative allele-preferential ASO designed to suppress mutant huntingtin protein (mHTT), aiming to slow or halt disease progression. Its allele-preferential nature means that VO659 preferentially binds to CAG repeats on the mutated allele while sparing a large portion of the wild-type allele, which is important for normal neuronal function.
“What sets VO659 apart is that it acts like a pipeline within a drug because of its ability to target multiple genetic neurological diseases, which is quite unique, as most genetic medicines are very specific to one disease,” Mr. Mackison says. “But in the case of the diseases we’re addressing—HD, SCA1, and SCA3, along with many other polyglutamine (polyQ) diseases—they are all caused by a common mutation, and that common mutation is an expansion of the CAG repeat in the gene of interest.”
Mr. Mackison clarifies that the CAG repeat expansion is the underlying mutation causing all polyQ diseases, of which there are nine in total. Currently, no disease-modifying therapies exist for polyQ diseases, resulting in early mortality. “Because we bind directly to the CAG repeat, we can have potential application broadly across all of these polyQ diseases. We have chosen to concentrate first on HD, SCA1, and SCA3, and that is where our current clinical trial is focused.”
HD is a rare, progressive genetic neurodegenerative disorder that typically begins in mid-adulthood and is ultimately fatal, Mr. Mackison says. It is characterized by movement disorders, cognitive impairment, and psychiatric manifestations such as depression and psychosis.
Mr. Mackison outlines that HD is caused by a CAG trinucleotide repeat expansion in the coding region of exon 1 of the HD gene (HTT), which leads to the production of mHTT with an elongated polyQ stretch at its N-terminus. This expanded stretch confers a toxic gain-of-function (GoF) to mutant proteins, resulting in widespread neuronal death. “Current therapies for HD are limited, alleviating only some movement and psychiatric symptoms. There is a significant unmet need for disease-modifying therapies,” he says.
SCA1 and SCA3 belong to a group of rare, progressive hereditary disorders that affect the cerebellum, brain stem, and spinal cord, Mr. Mackison says. More than 30 types of SCAs have been identified, with types 1 through 7 being the most common. SCAs are caused by translated CAG trinucleotide repeat expansions that encode polyQ stretches in disease proteins. The elongated polyQ stretches cause pathogenic effects via a dominant GoF mechanism, leading to the degeneration of specific neuronal subpopulations, which vary by SCA types.
“SCA1 and SCA3 present with ataxia of gait, stance, and limbs, along with dysarthria and oculomotor abnormalities. Currently, no disease-modifying therapies exist,” he adds.
Recently, VICO announced positive interim data in HD from its ongoing Phase 1/2a study of VO659 —the only clinical-stage program that directly targets the CAG repeat expansion causing HD.
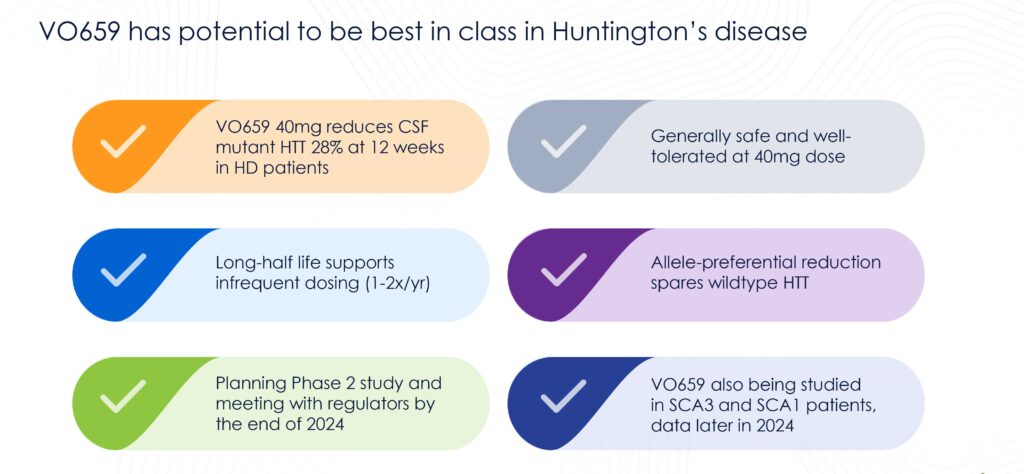
“We were very encouraged by immediate reductions in cerebrospinal fluid mHTT and no changes in Nf-L protein—two key HD biomarkers—in treated patients over the available follow-up period,” Mr. Mackison reports.
Results, first observed in HD patients, showed a 28% mean reduction in mHTT in cerebrospinal fluid at day 85 compared to baseline, with effects observed as early as day 29. VO659 was generally safe and well tolerated and has a long half-life, suggesting the potential for infrequent dosing—estimated at just 1-2 times per year.
“Given VO659’s long anticipated half-life, there is clear potential for this therapy to have an infrequent dosing schedule, and we look forward to exploring this further in clinical trials,” Mr. Mackison notes. “These data are a significant milestone for our team and for the HD community, which so desperately needs treatment options. We look forward to continuing the trial and advancing VO659 as quickly as possible for patients and their families.”
Mr. Mackison asserts that these interim data build on preclinical findings, where VO659 demonstrated potent target engagement across multiple disease models. Preclinical studies showed significant and dose-dependent reductions of mHTT and improved motor function in vivo in HD mouse models, along with allele-preferential reductions of mHTT in vitro in HD patient cell models.
Regarding SCA1 and SCA3, “We have not yet presented any data on our SCA patients but plan to do so at a future scientific meeting,” he adds.
Looking ahead, Mr. Mackison sees these interim Phase 1/2a data in HD as a success, and VICO plans to discuss advancing into Phase 2 clinical development of VO659 in HD with regulators by year-end, with an anticipated Phase 2 trial launch in 2025.
“It is our mission to bring safe, effective therapies to patients and families affected by devastating neurological diseases. These data represent an important milestone in our journey to realize this mission,” he concludes.
• • • • •
To connect with VICO Therapeutics or any other companies featured on BioTuesdays, send us an email at [email protected].