By Melane Sampson

Closely-held Creyon Bio is developing a faster, more cost-effective path to new medicines for rare and common diseases through the AI-enabled engineering of oligonucleotide-based therapies (OBMs) that deliver safe, effective precision medicine to the right cells at the right time.
“OBMs comprise a rapidly expanding category of drugs that have emerged as a proven super modality with vast potential to change the standard of care, address new opportunities for rare and common diseases, and actualize personalized medicine, especially as modern biology rapidly reveals the genetic and molecular basis of disease,” Christopher Hart, Ph.D., co-founder, CEO, and president of Creyon, says in an interview with BioTuesdays.
“Creyon’s technologies and efforts make engineering new OBMs to treat disease through these varied mechanisms, and perhaps yet to be discovered mechanisms, not only possible but also fast and efficient. We are even engineering delivery systems to directly target OBMs to specific cell types and tissues,” he says.
Dr. Hart explains that while OBMs have yet to reach their full potential, they are an ideal modality for creating gene-centric therapies. They include antisense oligonucleotides (ASOs), RNA interference (RNAi), and aptamers. Unlike small molecules or antibodies, OBMs are single- or double-stranded DNA or RNA molecules that bind via Watson-Crick-Franklin base pairing to enhance or repress the expression of target RNA, to treat or manage a wide range of diseases. Watson-Crick-Franklin hybridization is a zipper-like process that selectively and precisely binds to specific DNA or RNA positions within the cell.
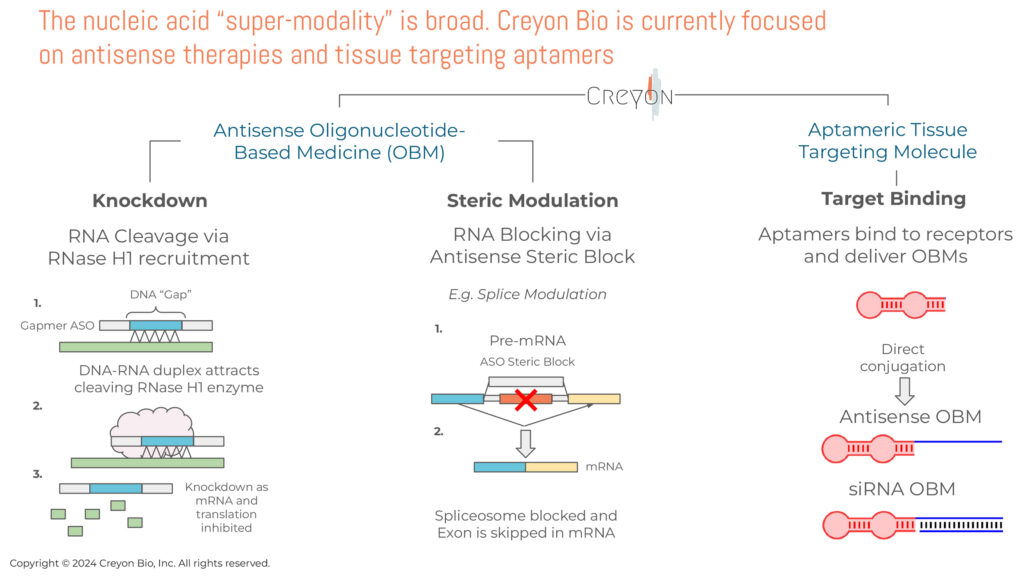
“In general, OBMs interfere or inhibit the RNA translational process within the cell and promote degradation of proteins associated with disease,” Dr. Hart points out. “As of the summer of 2024, there are 17 approved oligonucelotide drugs on the market, four of them are blockbusters, with hundreds more in development. What is very clear to us now is that these drugs can be exceptionally effective, with excellent target product profiles that allow for monthly, quarterly, or even every-six-month dosing, sometimes even administered in the patient’s home, and they are well-tolerated.”
OBMs can control the effective activity of genes through multiple mechanisms, and enhance or block molecular interactions, which can control how genetic information is used by the cell. This lays the groundwork for developing new medicines that directly target the genetic and molecular basis of disease—preventing toxic RNA or protein production, increasing levels of under-expressed enzymes, editing mRNA, or altering how the splicing machinery assembles genetic instructions that control protein production, he adds.
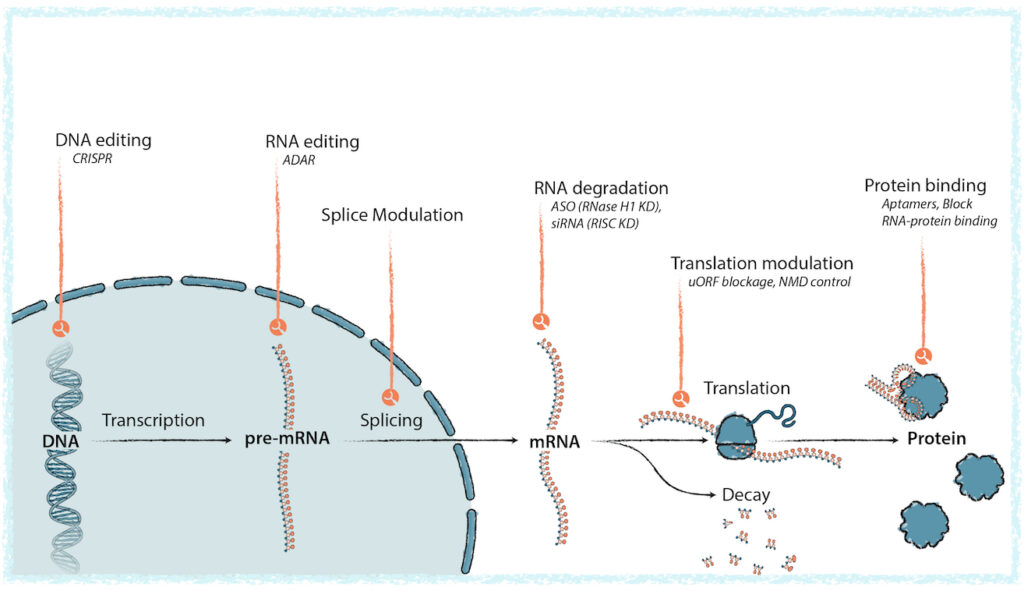
Creyon has built the first and only platform capable of engineering for safety first, creating novel OBMs with optimal pharmacological properties engineered to minimize side effects. “We are reimagining the way novel OBMs are created, transforming the process from drug discovery to drug engineering,” Dr. Hart contends.
Recently, in partnership with the TNPO2 Foundation, Creyon developed a novel allele-selective Locked Nucleic Acid (LNA) ASO treatment candidate for an ultra-rare and severe neurological disease caused by a single nucleotide variant in the Transportin-2 (TNPO2) receptor. An investigator-initiated clinical trial has received approval to dose a patient, and the study is ongoing, with initial results reported at the American Society of Gene and Cell Therapy (ASGCT) conference in 2024.
To demonstrate the power of its platform in this context, Creyon achieved significant milestones in the rapid development of ASO candidates. For its TNPO2 program, Creyon created 96 ASO candidates utilizing proprietary tools, AI, and custom datasets. Of those, 88 of the candidates were demonstrated to be safe utilizing in vivo and in vitro model systems, and several allele selective ASOs against the disease-causing TNPO2 variant were identified that demonstrated both safety and target selectivity in a cell model. The safest and most effective molecule was scaled up for GMP manufacturing, quality assessment, and compounding.
“Initial data from this trial demonstrated that the investigational treatment was successful in reducing seizures, restoring developmental milestones, and was well-tolerated through nine months,” Dr. Hart says. “These results illustrate a key pillar of our approach—to engineer safe drugs for rare and common diseases faster and more efficiently compared to traditional drug discovery techniques.”
Highlighting that predictive models were developed to guide molecular engineering from the outset to help mitigate potential safety concerns, Dr. Hart says this approach has enabled Creyon to rapidly identify a drug candidate to target a specific genetic variant and initiate a clinical study within a year. “This proof of concept underscores the transformative potential of AI in OBM drug engineering to rapidly create new OBMs for common and rare diseases and target the genetic underpinning of disease anywhere in the body.”
Dr. Hart outlines that the company has established three core competencies: a deep technology platform, a focus on engineering rather than discovering OBMs, and the ability to create tissue-targeting aptamers. “Creyon is highly differentiated, as our approach allows us to create best-in-class ASOs about one-hundred times faster than anyone else in the field, and we can unlock new therapeutic opportunities in tissues where others can’t practice.”
Since the company’s inception in 2019, funding of seed and series A has enabled Creyon to scale up to approximately 50 Creyoneers, with labs in San Diego, North Carolina, and Bangalore, India. “We have put together a pretty amazing team—highly interdisciplinary with a unique set of capabilities and deep capacity in chemistry and synthesis, pharmacology, and state-of-the-art data science.”
Looking forward, Dr. Hart says that the company is raising funds for the next phase of Creyon, focused on leveraging its technology stack to advance multiple programs forward and move them into the clinic. “We have one clinical program underway already, which will create multiple commercial opportunities, establish pharma partnerships, and continue to expand our core technologies, allowing us to broaden the use of OBMs even further.”
• • • • •
To connect with Creyon Bio or any other companies featured on BioTuesdays, send us an email at [email protected].