
Closely held VeriSIM Life is leveraging multi-disciplinary quantitative methods to predict patient outcomes and accelerate the discovery, development, and commercialization of transformative therapies. The company aims to eliminate inefficiencies and inaccuracies in translating drug candidates to clinical trials—moving beyond the traditional trial-and-error experimentation model.
“We believe our machine learning-driven approach will accelerate the development of medicines, getting them to market faster and improving patient outcomes—without relying on redundant trials or subjecting animals to the cruelty of testing,” Jo Varshney, Ph.D., founder and CEO of VeriSIM, says in an interview with BioTuesdays.
“Unlike conventional development methods, our platform isn’t constrained by disease-specific or target-based limitations. This enables us to explore a wide range of therapeutic areas and expedite the development of breakthrough treatments,” she adds.
Dr. Varshey recounts how her frustration with inefficiencies in the drug development space led her to establish VerSIM Life in 2017. Together with her team of dedicated scientists, modelers, chemists, and software engineers, she is committed to transforming healthcare through better predictive models.
“With my background in both veterinary and human health, animal testing has never sat well with me. We create synthetic diseases in animals that live in highly controlled environments—unlike the complexities of real human conditions,” she contends.
“Animal testing has not really changed in more than a century. This year, the FDA announced plans to phase it out in large molecule testing—and for good reason. It’s expensive and often poorly translatable to humans due to inherent biological differences.”
Dr. Varshney believes a better way forward is combining computational modeling with biology, “where biology remains the first principle and driver over the AI piece.”
To achieve meaningful scale and impact across diverse patient populations and drug types, Dr. Varshney says she needed to go big and that resulted in the formulation of VeriSIM Life. “I wanted to solve this challenge of how we look at translatability between non-clinical and clinical trials and make it better, more efficient, and safer so more drugs are successful.”
Dr. Varshney explains that the challenge was to create AI-based translational models that could help identify potential clinical risks that could be seen in patients, before the drug entered into clinical trials. “It traditionally takes more than $6 billion and 12 to 15 years to successfully bring a drug to market—which if you do the math means fewer innovations, longer cycles of clinical trials, and really very few drugs approved and available to patients. We needed an integrated solution to change that.”
To meet this challenge, VeriSIM developed a first-in-class, drug decision engine called BIOiSIM, which delivers earlier, actionable insights—enabling faster drug discovery and de-risking of R&D.
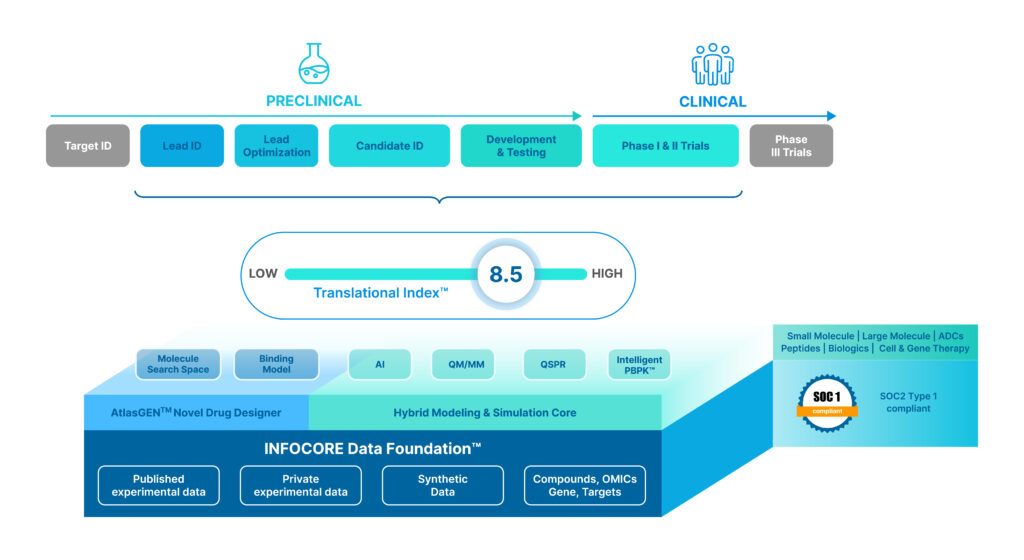
Dr. Varshney points out that the BIOiSIM platform combines a vast chemical search space, thousands of validation data sets, multi-compartmental models, machine learning (ML), deep learning, and large language models. This fusion enables unprecedented chemical and biological relevance in drug design—agnostic to indication or modality.
BIOiSIM helps research scientists avoid the design and pursuit of dead-end drug candidates by identifying those with high clinical potential from day one—while offering the flexibility of being able to pivot and quickly adapt to address translation challenges.
“There are countless brilliant scientists out there and BIOiSIM gives them superpowers—the ability to make smarter decisions earlier and increase their chances of clinical successes,” Dr. Varshney says.
The BIOiSIM platform reduces the time it takes to get to Investigational New Drug (IND) status by an average of two and a half years. Its robust AI/ML engine reduces the need for unnecessary in vivo experimentation, representing a cost savings of $3 million compared to conventional testing.
The VeriSIM team has simulated various species—including humans—creating digital twins to study drug behavior across patient populations. This simulation-based triage helps researchers determine whether animal studies are necessary at all—reduce, refine, or replace animal testing.
“We analyze historic challenges—from R&D to clinical trials—then codify and simulate past outcomes with different types of drugs: novel drugs, molecules, approved drugs, or failed drugs, so that AI can learn from these interactions. This creates a kind of mega-map that researchers can use to avoid repeating mistakes,” Dr. Varshney says.
Dr. Varsney emphasizes that BIOiSIN was designed to augment—not replace—human experience. One example is VeriSIM’s collaboration with the Mayo Clinic, where highly experienced research physicians contribute real-world clinical insights and critical knowledge that AI alone can’t generate.
“The experts at Mayo Clinic understand not only the disease but also patient experiences, which really helps us build better models within BIOiSIN. They also know about the negative data—things that didn’t work out in the clinic or in research—and that’s golden information for an AI company because those things don’t get published and they have more lessons learned than successful stories, which are so few and far between,” she says.
To keep BIOiSIN outcome focused, VeriSIM created a Translational Index—a sort of credit score for drug candidates. It helps partners identify challenges early on to improve clinical viability before entering trials.
“From a financial perspective, this means less time lost to trial-and-error, fewer repeated studies, and better allocation of resources. It also increases the probability of success and enables quicker pivots if needed. It’s a win-win-win for developers, investors, and—most importantly—patients who need drugs yesterday,” Dr. Varshney says.
VeriSIM not only supports external drug developers with IND-enabling programs, but it also uses its platform internally. The company is currently advancing a portfolio of assets, particularly in rare diseases.
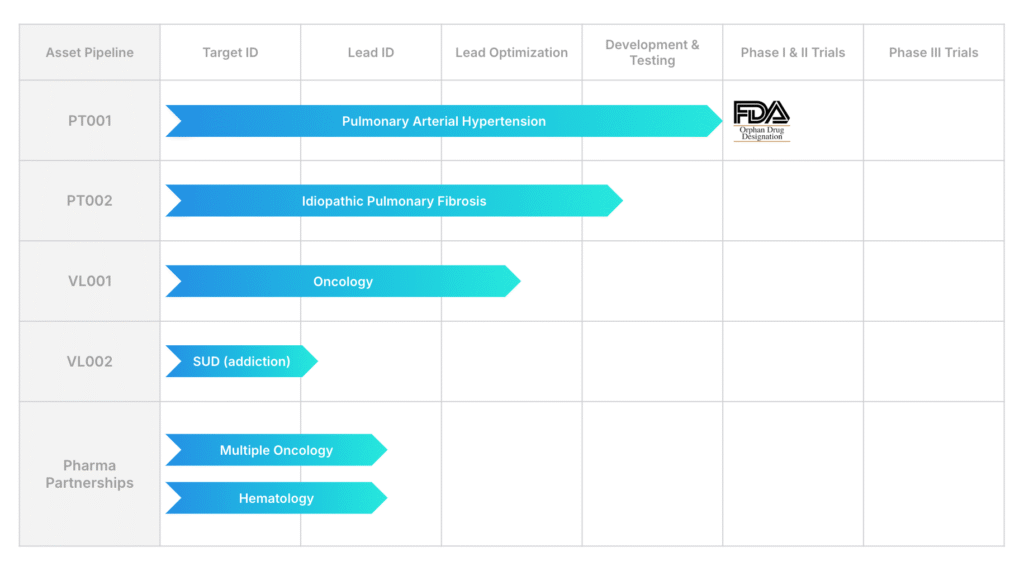
“As the first-to-market in this space, we believe it’s essential to validate the technology ourselves. We’re applying it to high-need areas like pulmonary arterial hypertension, substance use disorders, and oncology,” Dr. Varshney says.
In each program, VeriSIM leverages its predictive engine to prioritize candidates with the highest chance of success, working closely with pharma and regulatory partners to ensure its scoring system translates effectively in real-world settings.
By building a scalable, AI-enabled drug development pipeline, the company is expanding into multiple therapeutic areas, including fibrosis, hepatocellular carcinoma, addiction (in partnership with the NIH), and metabolic diseases.
“There is a better way to develop drugs—and we’re among the first to enable ROI-driven development,” Dr. Varshney concludes. “While we can’t promise success every time, we can identify clinical risks earlier and help researchers fast-track their innovations to where they’re needed most—and we are very excited to be on this journey with them.”
• • • • •
To connect with VeriSIM Life or any other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.







