
Eledon Pharmaceuticals (NASDAQ: ELDN) is pioneering a potential breakthrough in immunosuppression therapy aimed at extending the longevity of transplanted organs and enhancing the overall health and quality of life for transplant recipients—disrupting a field that has seen little innovation in the past 30 years.
“Our focus is on extending the functional life of organ transplantations, which is often the last option for patients who are suffering from diseases that leave them with no other choice but to receive a new organ to survive,” David-Alexandre Gros, MD, CEO and board member of Eledon, says in an interview with BioTuesdays.
The company’s lead compound, tegoprubart, is an anti-CD40 Ligand (CD40L) antibody with high affinity for CD40L—a well-validated biological target with broad therapeutic potential to regulate the immune system’s response and prevent organ transplant rejection.
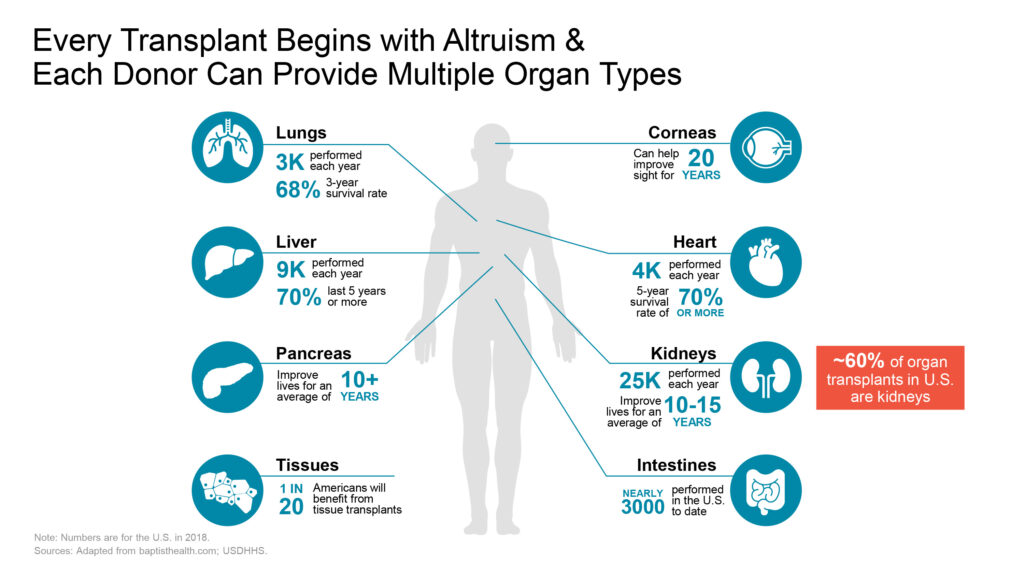
“The most common type of transplantation is kidney transplantation, making it critical to improve outcomes for kidney transplant patients, as waiting lists—often four to five years long—are expected to double in the next decade,” Dr. Gros asserts. “The alternative—dialysis—not only places a huge burden on the patient but it often has poor survival rates and is extremely costly.”
He adds that in the U.S. alone, approximately 100,000 people are currently waiting for a kidney transplant, and for every five successful transplants, one patient dies while waiting.
The current standard of care, tacrolimus, a calcineurin inhibitor approved in 1994, is effective in preventing transplant rejection but comes with severe drawbacks, Dr. Gros points out. “The irony is that while tacrolimus is very good at protecting the transplanted organ, it is nephrotoxic—meaning it damages the kidneys. It induces hypertension and diabetes, two leading causes of kidney failure. It can also cause central nervous system side effects, including tremors and brain fog,” he adds.
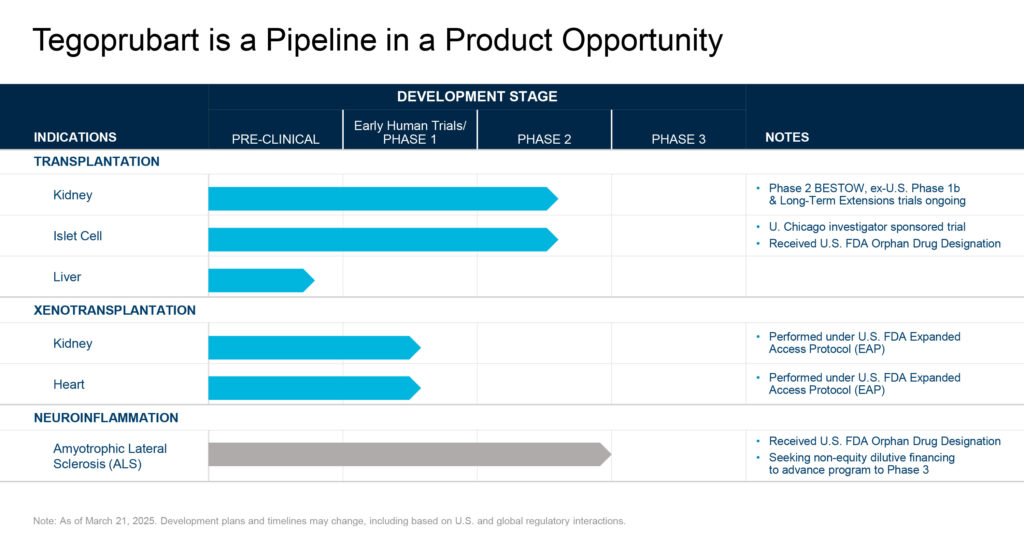
Eledon is leveraging extensive historical research on the anti-CD40L biology to advance tegoprubart through preclinical and clinical studies in different types of transplantation including kidney allograft (human-to-human) transplantation, xenotransplantation (animal-to-human), islet cell transplantation, and liver transplantation as well as in amyotrophic lateral sclerosis (ALS).
The CD40L pathway is a promising target for enhancing transplant function and longevity, Dr. Gros explains. “The central role of CD40L signaling in driving pro-inflammatory responses makes it an attractive candidate for therapeutic intervention—not only for preventing transplant rejection but also for modulating immune responses in autoimmune diseases and neuroinflammation.”
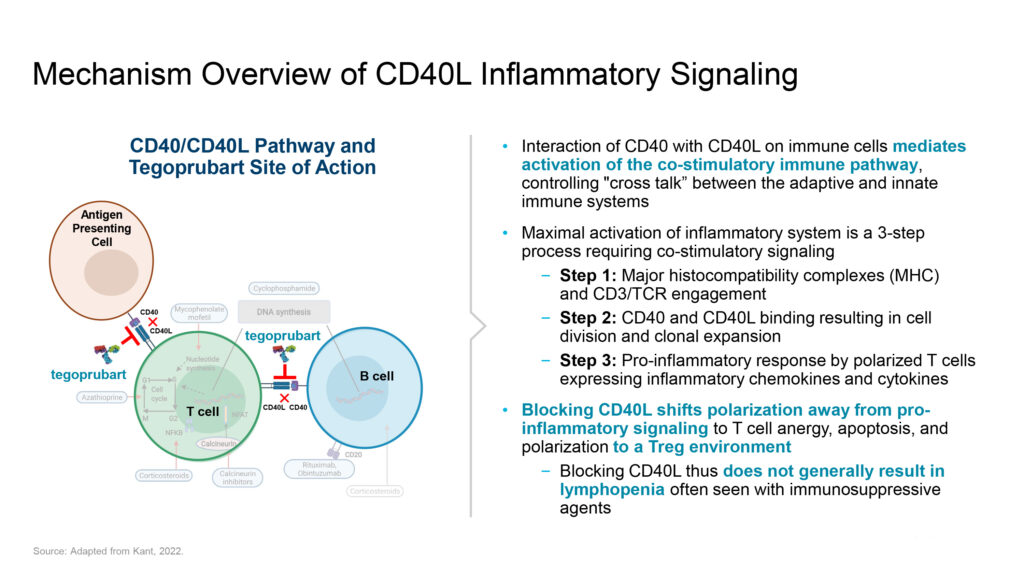
“Blocking activation of the CD40L pathway has been shown to slow disease progression and pathology in preclinical models of autoimmunity and neurodegeneration while also preventing both acute and long-term transplant rejection across multiple animal species,” he says.
The interaction of CD40 with CD40L on immune cells mediates the activation of the co-stimulatory immune pathway, disrupting the excessive signaling between the innate and adaptive immune systems that fuels an uncontrolled inflammatory response, Dr. Gros points out. The engagement of these receptors also plays a pivotal role in immune system activation by regulating both antibody and cellular immune response.
“Targeting the CD40L pathway may provide significant advantages over current treatment options, including the ability to control immune response and increase transplant durability with fewer side effects,” he adds.
Emphasizing that the average kidney transplant recipient experiences kidney failure within 10 to 15 years post-transplant, Dr. Gros says, “If someone is in their 20s or 30s, that’s not a lot of time. To live a normal lifespan, they will need multiple organ transplants.”
He highlights that Eledon is striving to both extend the functional life of transplanted organs and reduce the serious side effects associated with current immunosuppressive treatments such as tacrolimus.
The company has initiated three parallel clinical trials to evaluate tegoprubart for the prevention of organ rejection in patients receiving a kidney allotransplant. A Phase 1b open-label, single-arm trial, a Phase 2 long-term safety and efficacy extension study, and the international BESTOW Phase 2 head-to-head superiority study comparing tegoprubart with tacrolimus. The BESTOW multicenter, two-arm, active comparator study has enrolled over 120 participants across North America, Europe, and Latin America to evaluate the safety, pharmacokinetics, and efficacy of tegoprubart compared to tacrolimus, reaching its target enrollment approximately four months earlier than originally planned. Dr. Gros says patients who complete this trial have the option to enroll in the long-term extension study of tegoprubart to remain on therapy and continue to be monitored.
Additionally, Eledon is supplying tegoprubart for an investigator-led trial at the
University of Chicago Medicine Transplant Institute. The trial is evaluating pancreatic islet transplantation in patients with T1D.
In October 2024, Eledon announced early data from the UChicago Medicine trial in islet transplantation trial demonstrating potentially the first-ever human cases of insulin independence using an anti-CD40L monoclonal antibody therapy without the use of tacrolimus, the current standard of care for prevention of transplant rejection. Among the three trial participants who received islet transplant, at the time of the data readout two were functionally cured of T1D—achieving insulin independence and normal hemoglobin A1C levels, a measure of average blood glucose, post-transplant. The third patient, who recently received an islet transplant at the time of the announcement, reduced insulin use by more than 60% and showed good glucose control within three days following the procedure.
“Combined with the promising results in kidney allotransplant procedures as well as heart and kidney xenograft procedures we are also involved with, these data from subjects following islet transplantation demonstrate tegoprubart’s potential to protect transplanted organs and cells while being generally well tolerated, with no unexpected adverse events, severe hypoglycemic episodes, or graft rejection,” Dr. Gros contends.
He points out that all transplant recipients require lifelong immunosuppression therapy to prevent organ rejection. “It’s a large market from a dollar perspective, which is evident with tacrolimus since it still generates approximately $1.5 billion in annual global revenue—30 years after launch—which is remarkable and underscores the significant unmet need.”
Dr. Gros notes that while Eledon is a small biotech company, there are only approximately 250 transplant centers in the U.S. and similarly few in other countries. With a team of about 25 sales representatives, he believes the company would be well positioned to cover the immunosuppression drug market in terms of ultimately commercializing tegoprubart following a potential regulatory approval.
“This past year was transformative for Eledon,” Dr. Gros suggests. “We achieved multiple key clinical milestones across kidney, islet cell, and xenograft transplantation, reinforcing tegoprubart’s potential as a best-in-class immunosuppressant.”
Looking ahead, Dr. Gros says, “Our ultimate goal is to be the next cornerstone immunosuppression therapy in transplantation regardless of the transplant type—whether human kidney, heart, lung or islet cell—and regardless of the source of the organ—whether deceased donor, living donor, or stem cell-derived, or in the case of xenotransplant, a genetically-modified pig.”
He adds, “Our mission is simple: we want to transform lives with one transplant for life, so that every donor’s gift keeps on giving.”
• • • • •
To connect with Eledon Pharmaceuticals or any other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.







