Closely held Tasman Therapeutics is shifting the treatment landscape for treatment-resistant depression (TRD) with its registered, proprietary formulation—a first-of-its-kind, oral extended-release ketamine tablet.
“Our goal is to deliver a safe, convenient, self-administered, at-home solution that enables the rapid and lasting reduction of depressive symptoms in individuals with difficult-to-treat depression,” Dan Browne, CEO of Tasman, says in an interview with BioTuesdays.
“Our lead candidate, R-107, a racemic ketamine extended-release oral tablet, is in clinical development and well positioned as a potential blockbuster product in the neuropsychiatric market, with sales projected to reach approximately $2 billion,” he adds.
Mr. Browne emphasizes that in the U.S. alone, 60 million patients suffer from major depressive disorder (MDD)—with 20 million classified as having TRD. This patient population faces higher risk of suicide. TRD is often defined as depression unresponsive to at least two anti-depressant therapies.
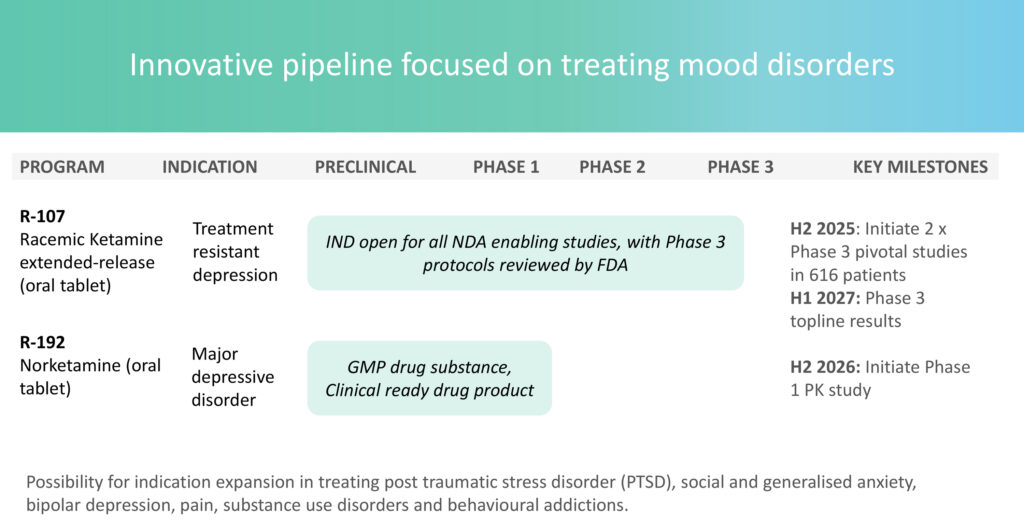
Tasman is also developing a second candidate, R-192, a norketamine oral tablet for MDD. Clinically ready, the company expects to initiate its Phase 1 pharmacokinetic study of R-192 in the second half of 2026.
Mr. Browne explains that current treatment options for TRD are often invasive, poorly-tolerated, and require in-clinic administration with extensive monitoring for adverse effects, including dissociation—a loss of connection between thoughts and feelings often described as a ‘trip’ or detachment from reality.
“The current gold standard in TRD care is electroconvulsive therapy (ECT)—which requires anesthesia and electrical stimulation—or SPRAVATO, an esketamine nasal spray by Johnson & Johnson (NYSE: JNJ), both of which must be administered under the supervision of healthcare providers in a clinical setting,” he says.

Joining the BioTuesdays interview is Peter Surman, Ph.D., CSO of Tasman, who, together with Professor Paul Glue, CMO of Tasman, developed R-107 technology in the labs of closely held Douglas Pharmaceuticals, Tasman’s New Zealand-based parent company. Tasman is a newly formed, U.S.-based subsidiary of Douglas—New Zealand’s largest pharmaceutical firm—and will be headquartered in the San Francisco Bay Area as an independently financed company.
Dr. Surman highlights that ketamine has been used for decades off-label as a treatment for mood disorders. “Unlike traditional antidepressants, which can take up to six weeks to work, ketamine and esketamine target GABA and glutamate neurotransmitters via different biological pathways. We’ve found that patients who don’t respond to conventional treatments often respond rapidly to these agents—sometimes within one to two days, with many achieving remission within a week.”
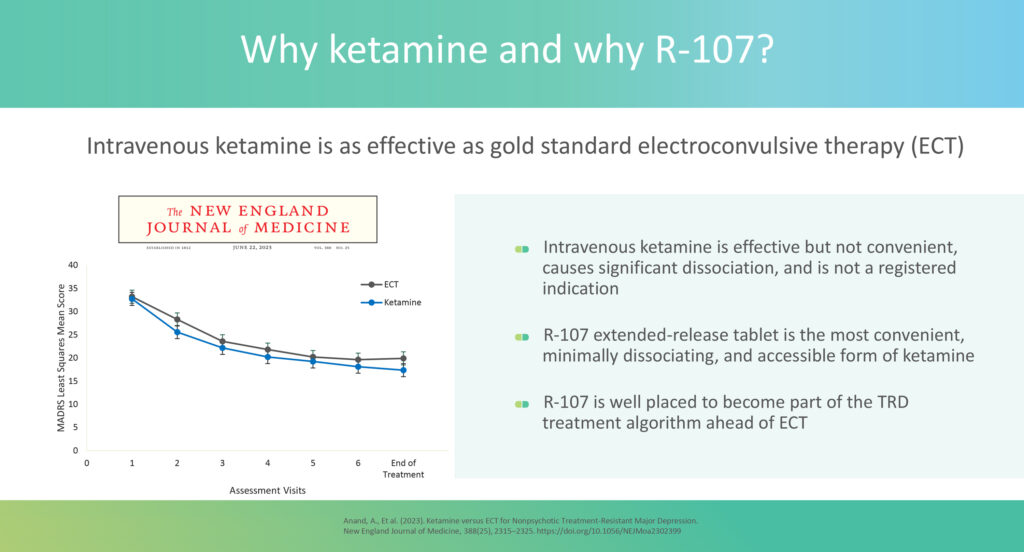
However, Dr. Surman points out that IV or intranasal administration methods cause ketamine or esketamine to be rapidly absorbed into the central nervous system, leading to high peak concentrations in the blood. This often results in acute dissociation, making it unsafe for patients to be unmonitored. As a result, they must remain in a certified medical clinic for at least two hours post-dose, during which their dissociative symptoms as well as oxygen levels and blood pressure are closely monitored due to significant spikes in blood pressure and possible respiratory depression. This level of supervision means patients spend nearly half a day in the clinic, once or twice a week.
In contrast, R-107 offers a self-administered, at-home alternative, significantly enhancing access and dignity for individuals living with hard-to-treat depression. “To date, more than 200 patients have been treated with R-107 without significant dissociation. The median score on the Clinic-Administered Dissociative States Scale (CADSS) was zero for R-107 in Phase 2 trials, where only scores above four are considered clinically significant,” Dr. Surman notes.
“By delivering ketamine orally and slowing its release over several hours, R-107 achieves a lower peak blood concentration of ketamine—the component responsible for dissociation—while maintaining enough exposure to ketamine to produce a rapid and robust antidepressant response, as confirmed in our Phase 1 and 2 studies,” he adds.
In the randomized, placebo-controlled Phase 2 BEDROC dose finding study, R-107 demonstrated rapid and sustained antidepressant efficacy with minimal dissociation and blood pressure fluctuations. Approximately 75% of TRD patients achieved remission within one week of treatment initiation, and the 180 mg dose group showed a statistically significant 6.1-point reduction in depression symptoms assessed by the Montgomery-Åsberg Depression Rating Scale (MADRS) compared to placebo at the 12-week endpoint. The results were published in Nature Medicine in March 2024.
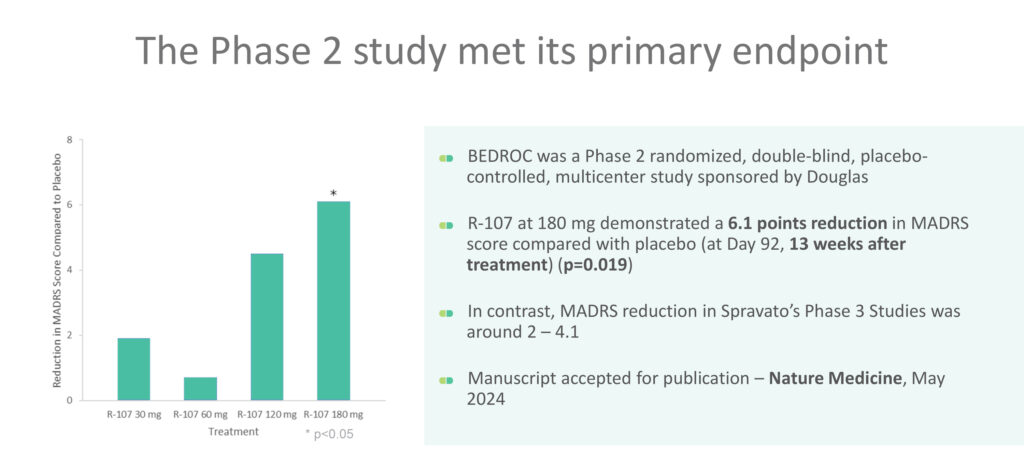
“Patients in our Phase 2 study had failed on average to respond to five traditional anti-depressants—some had struggled with depression for one or two decades with no relief because nothing was working for them,” Dr. Surman contends. “We had a fantastic response rate with almost half of our patients in remission and more than 90% requesting to carry on with R-107 in the six-month open label safety extension study, now under review by Cell Reports Medicine.”
Tasman’s pivotal Phase 3 ROCKET Program is set to begin enrolling patients in the second half of 2025. This study will provide critical data on safety, onset of effect, and durability of R-107. The company plans to present findings to the FDA in 2027.
To support its global Phase 3 program, Tasman is currently conducting a $175 million Series A fundraising round, targeting the U.S., EU, and other international markets.
Looking ahead, Mr. Browne says the company is encouraged by the possibility of expedited regulatory approval in the U.S. and EU, with potential market entry as early as 2028, and a strong IP protection in place with patents through 2037 and beyond.
“There are no shortages of opportunities for both R-107 or R-192—including potential indication expansion into PTSD, social and generalized anxiety, bipolar depression, pain, substance use disorders, and behavioral addictions,” he notes.
In closing, Mr. Browne reflects, “We are all connected to someone who has been consumed by depression. Tasman has the power to transform lives, with a safe, rapid, and effective medication that patients can take in the comfort of their own homes—one that brings hope, healing, and lasting change.”
• • • • •
To connect with Tasman Therapeutics or any other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.