By Melane Sampson

MaaT Pharma (EURONEXT:MAAT) is at the forefront of developing the world’s first full-ecosystem approach to restoring microbiome symbiosis in oncology, improving cancer patient survival through innovative, fecal-derived therapies designed to modulate the immune system and restore gut balance.
“We founded MaaT Pharma with the mission of extending the lives of cancer patients. To achieve this, we have developed a novel class of microbiome therapies that address critical medical needs in oncology. As one of the few end-to-end microbiome companies, we oversee the entire process—from bioprocessing donor-derived and co-cultured drug candidates to rigorous screening and large-scale GMP-compliant manufacturing, the largest of its kind in Europe,” Hervé Affagard, co-founder and CEO of MaaT Pharma, says in an interview with BioTuesdays.
MaaT Pharma’s Microbiome Ecosystem Therapies (MET) platform offers high-diversity, high-richness, and indication-specific microbiome-based products designed to harness the functional diversity of a large microbiome ecosystem.
Using proprietary pooling and co-cultivation technologies, MaaT Pharma develops therapies that address urgent unmet medical needs. “Pooling fecal microbiota from multiple healthy donors allows us to double the richness and diversity, while reducing product variability by fivefold compared to single donor approaches,” explains Mr. Affagard. “We adhere to the highest regulatory standards to ensure safety and maximize quality through our patented processing methods, which guarantee high traceability and the preservation of bacterial strains.”
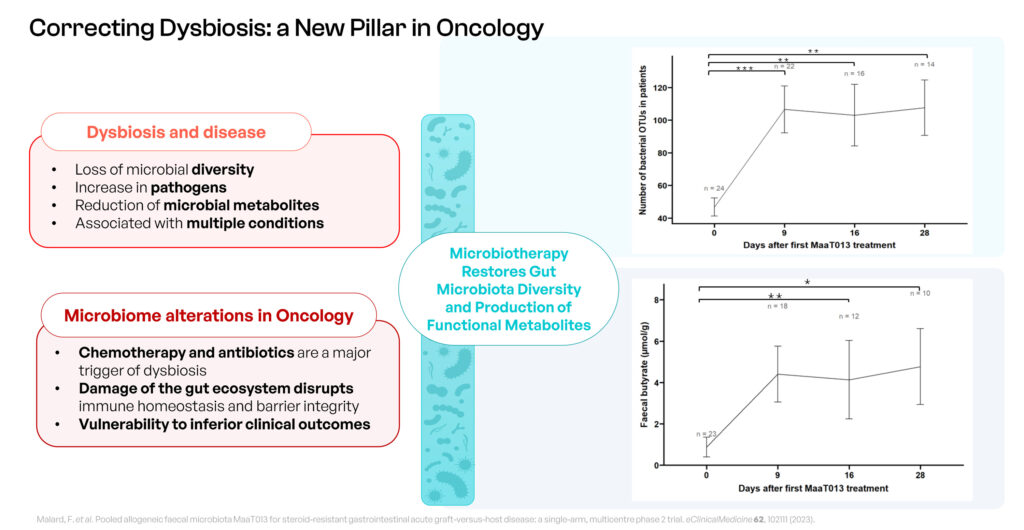
The company’s lead product candidates, MaaT013 and MaaT033, fall under the MET-N category—where N denotes native. According to Mr. Affagard, MET-N donor-derived products aim to restore gut symbiosis in cancer patients whose microbiota are severely compromised, often due to chemotherapy. “Our MET-N products prioritize safety, maximize richness and diversity, and ensure the presence of anti-inflammatory species in the gut,” he explains.
MaaT Pharma’s pipeline employs two primary approaches: immune-oncology and hemato-oncology. In immune-oncology, the therapies aim to restore and modulate the microbiome to enhance patient responses to immune checkpoint inhibitors (ICI) for solid tumors. In hemato-oncology, the company targets graft-versus-host disease (GvHD), a severe complication of allogeneic hematopoietic stem cell transplantations (allo-HSCT), where grafted cells attack the host.
“GvHD carries a high mortality rate and has limited treatment options. It wasn’t until 2019, more than 60 years after the first stem cell transplantation, that a treatment for graft rejection—ruxolitinib—became available. However, some patients are intolerant to ruxolitinib leaving them to face an urgent unmet medical need,” Mr. Affagard notes.
Patients with steroid-refractory GvHD or ruxolitinib intolerance face one-year survival rates as low as 15%.
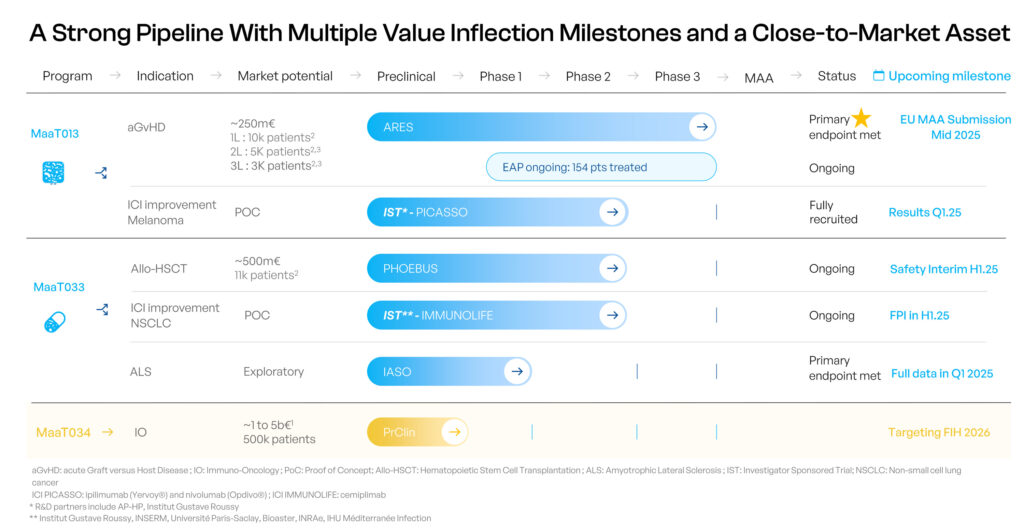
MaaT013 is currently in a pivotal Phase 3 trial, ARES, for patients receiving Allo-HSCT, with acute GvHD involving gastrointestinal involvement (GI-aGvHD), who are refractory to steroids and ruxolitinib. ARES is the first global Phase 3 trial for a microbiome-based therapy in hemato-oncology.
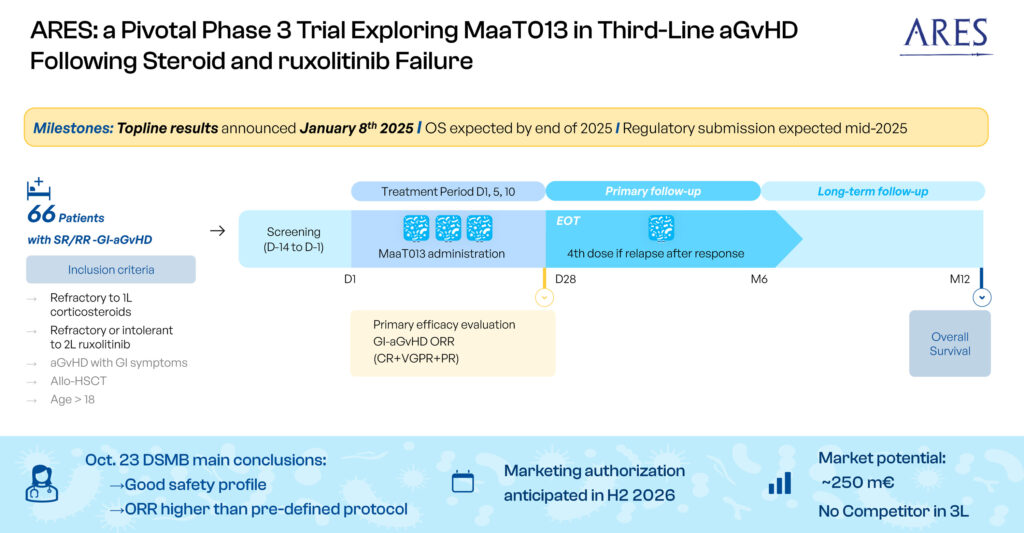
Both the FDA ad the EMA have granted MaaT013 orphan drug designation. This donor-derived, high-richness, high-diversity product incorporates Butycore, a proprietary group of bacterial species that produce anti-inflammatory short-chain-fatty acids. MaaT013 is designed to restore microbiome functionality and address the effects of stressors such as antibiotics and chemotherapy, directly targeting the gut-immune interface.
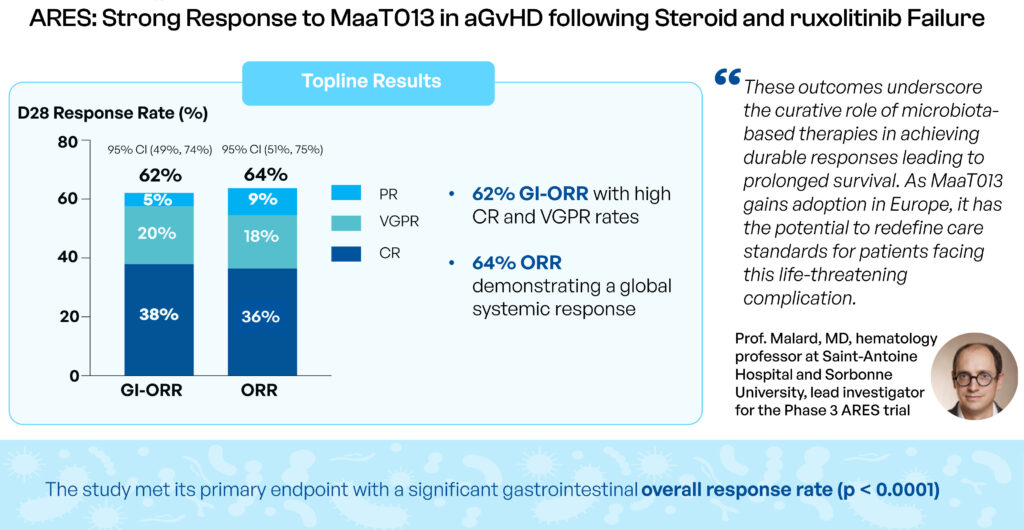
In early 2025, MaaT Pharma announced that its Phase 3 ARES study met its primary endpoint, achieving a significant gastrointestinal response rate of 62% at day 28—well above the expected 38%. “These results are groundbreaking, positioning MaaT013 as a potential first-in-class third-line treatment for GI-aGvHD,” Mr. Affagard asserts.
MaaT033, an oral, freeze-dried, multi-donor-derived formulation, is another high-richness, high-diversity product containing Butycore. It is under development as an adjunct therapy for patients undergoing allo-HCT and other cellular therapies.
“Allo-HCT is a potentially life-saving procedure for blood cancer patients, such as those with acute myeloid leukemia. However, antibiotics and chemotherapy often deplete the gut microbiota, increasing the risk of GvHD disease and infection, with high associated mortality rates,” Mr. Affagard outlines.
Granted orphan drug designation by the EMA, MaaT033 is currently in the international Phase 2b PHOEBUS trial, evaluating its efficacy in improving 12-month overall survival in blood cancer patients undergoing allo-HCT. “PHOEBUS is the world’s largest randomized controlled trial for a microbiome therapy in oncology,” he adds.
Additionally, MaaT033 is in a Phase 1b study for Amyotrophic lateral sclerosis (ALS). “We expanded our research to ALS as there is clinical evidence suggesting a link between gut microbiota and the disease, indicating a potential disease-modifying role for the microbiome,” Mr. Affagard contends. With no cure available, ALS remains a fatal condition with a life expectancy of three-to-five years.
While MaaT Pharma has demonstrated proof-of-concept with its native products, Mr. Affagard highlights the need for scalable manufacturing for larger indications. To meet this demand, the company developed MET-C, a unique, patented co-culture technology, powered by AI-driven analysis and advanced validation methods. These MET-C candidates replicate the richness and diversity of native microbiome ecosystems while tailoring products for specific indications at industrial scale.
The MET-C portfolio includes MaaT03X and MaaT034. MaaT03X is a candidate composed of hundreds of co-cultured species, encompassing both supportive and functional microbial networks tailored to specific indications. MaaT034, a first-in-class co-cultured product, aims to optimize intestinal microbiome functions before and during immunotherapy across major oncology indications. It seeks to become a tumor-agnostic adjunctive therapy to ICIs, improving survival outcomes for cancer patients.
The company’s proprietary AI-powered drug discovery and analysis platform, gutPrint, serves as the core engine for product design and development, driving the creation of new MET candidates. “gutPrint integrates data from healthy donors, clinical studies, literature, and microbiome databases to identify functional microbial networks relevant to specific diseases,” Mr. Affagard says. “The platform then designs METs to enhance clinical outcomes while monitoring drug development and manufacturing processes.”
“MET candidates undergo validation through disease-relevant in vitro assays and in vivo models, refining the final microbial composition,” Mr. Affagard explains. “Each step enriches our database, deepening insights into the host-microbiome relationship.”
Looking forward, Mr. Affagard envisions MaaT Pharma as a global leader in immune modulation, revolutionizing patient care through innovative therapies powered by microbiome science. “We believe microbiome therapies are revolutionary, and we are committed to rallying others around this mission to spread the message of their transformative potential,” Mr. Affagard concludes, emphasizing MaaT Pharma’s unwavering dedication to pioneering a new era in oncology.”
• • • • •
To connect with MaaT Pharma or any other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.