By Melane Sampson

Vivos Therapeutics (NASDAQ:VVOS) is developing and commercializing innovative diagnostic and treatment methods for adult and pediatric patients suffering from sleep-related breathing disorders, including severe obstructive sleep apnea (OSA) resulting from inadequate cranial-facial development.
“Unlike CPAP or other nonsurgical OSA treatments that require a lifetime of nightly intervention, our proprietary Vivos CARE method is the first nonsurgical, noninvasive, non-pharmaceutical, FDA-approved oral appliance option for a potentially lasting rehabilitation of severe OSA in adults and children aged six to 17—with an average treatment time of about 12 months,” R. Kirk Huntsman, co-founder, chairman, and CEO of Vivos says in an interview with BioTuesdays.
Mr. Huntsman points out that OSA is a serious sleep disorder that occurs in adults and children whose cranial-facial development is inadequate to allow for an optimal airway. OSA occurs when a blockage in the airway causes a lack of oxygen, which activates a survival reflex that wakes the sleeper just enough to resume breathing, interrupting the sleep cycle any number of times during the night and preventing restful sleep.

“Over the past 20 years, scientific research has shown that there is a clear link between OSA and more than 40 different chronic, life-threatening diseases, including cancer, Type 2 diabetes, cardiovascular disease, fibromyalgia, stroke, hypertension, dementia, and mental health issues, among others,” Mr. Huntsman contends.
In children, OSA has been linked to ADD, ADHD, bedwetting, lower IQ, chronic allergies, aggression, and delayed puberty. However, many parents are unaware of the options that exist to help these conditions, which are often caused by sleep disorders, including OSA, he adds.
Vivos Guide appliances guide the growth and development of the hard and soft tissues essential to the formation of a fully functioning airway. Early intervention may help prevent OSA in adults.
Following the 2023 FDA approval of Vivos CARE oral medical devices for the treatment of mild-to-severe OSA in adults, Vivos was granted FDA approval to treat children with moderate-to-severe OSA in September 2024. “This is the first time any oral appliance has been FDA-cleared to treat any level of OSA in children,” Mr. Huntsman emphasizes.
An estimated 10 million children in the US alone, and more than 1 billion people worldwide, suffer from breathing and sleep disorders, including OSA. Mr. Huntsman cites the lack of knowledge within the medical and dental professions, as well as complex testing, diagnosis, referral, and treatment planning as contributing to the fact that more than 80% of OSA patients remain undiagnosed, untreated, and unaware.
Highlighting that new technologies and home sleep testing products have presented a breakthrough in the testing and diagnosis of OSA, Mr. Huntsman says, “In the past, testing had to be done overnight in a sleep lab using polysomnography at a cost of up to $4,000 per night. Today’s technology enables accurate sleep tests to be done at home using devices that costs under $100, significantly reducing the barrier to determining who is at risk for OSA.”
“OSA and its related health effects burden the U.S. economy by approximately $300 billion annually,” he adds.
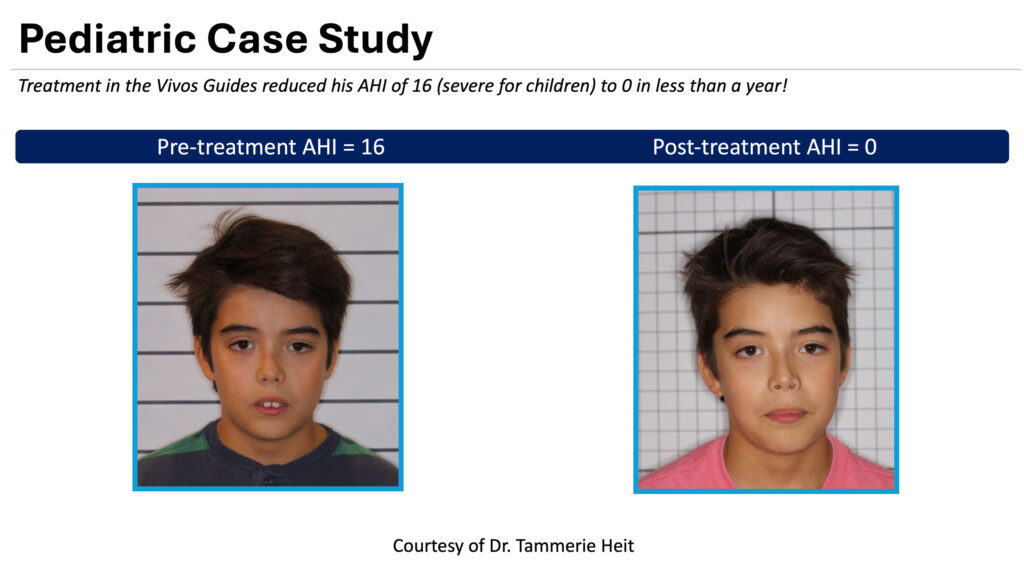
The Vivos method includes treatment regimens that employ its proprietary Vivos CARE oral appliance therapy and other modalities that alter the size, shape, and position of the jaw and soft tissues that comprise a patient’s upper airway and palate. The Vivos method opens airways, aiming to significantly reduce symptoms and conditions associated with mild-to-severe OSA in adults and moderate-to-severe OSA in children, serving to lower the Apnea Hypopnea Index (AHI) score.
AHI is used to indicate the severity of sleep apnea based on the number of apnea and hypopnea events—when breathing stops—per hour of sleep. Apnea is the complete absence of airflow through the nose and mouth. Hypopnea is overly shallow breathing or an abnormally low respiratory rate and is defined by a decrease of air entering the lungs. According to the American Academy of Sleep Medicine, AHI is categorized as mild (five to 15 events per hour), moderate (15 to 30 events per hour), and severe (more than 30 events per hour).
Mr. Huntsman explains that Vivos is leveraging tried-and-true dentistry techniques that have been implemented for decades, applying modern technology to reshape the oral cavity and widen the airway that sits right behind it. “We are actually redeveloping and enhancing the human airway.”
He adds that in addition to being non-invasive, non-surgical, non-pharmaceutical, and pain free, the Vivos approach is cost-effective compared to CPAP and other non-surgical OSA treatments and implants. “Our Vivos treatment approach costs between $8,000 and $10,000—a one-time cost, while CPAP’s total lifetime cost is approximately $38,000. Traditional lifetime oral appliance therapies range from $17,500 to $28,000. Inspire Medical’s implant, which stimulates tongue protrusion to keep airways open during sleep, can cost patients between $40,000 and $80,000.”
Vivos is the only company with proven safe solutions for diagnosis, prevention, management, and rehabilitation of OSA. “Before we came along, no one in history had ever attempted the complete resolution of OSA, non-surgically,” Mr. Huntsman says.
“We have now treated more than 60,000 patients worldwide, helping them to breathe better, sleep better, and feel better,” he adds.
Mr. Huntsman outlines that two years ago, the company made a strategic decision to pivot from a heavy reliance on dentists enrolling in the training and sales of Vivos devices for their patients to a new focus on the strategic affiliations and acquisitions of dedicated medical sleep clinics and professionals. He anticipates that this new business model will significantly drive revenue and margin growth while lowering operating costs across the board.
Recently, the company identified a major strategic affiliation opportunity with Rebis Health (Colorado Sleep Institute), which is now active and is expanding operations into two additional locations. “This alliance will put us in touch with tens of thousands of patients, which we are very excited about,” Mr. Huntsman says.
“We have high-margin products and first-mover advantage with a clear technological edge and multiple one-of-a-kind regulatory clearances to execute an aggressive go-to-market—first in Colorado, and then nationwide via telemedicine platform,” he asserts.
“OSA is a complex disorder, but there is a growing awareness worldwide about the importance of unobstructed breathing and sound sleep on overall health and well-being,” Mr. Huntsman concludes. “More and more patients are seeking treatment for sleep disorders. We believe that Vivos is at the epicenter of addressing the many chronic illnesses linked to OSA that are plaguing humanity today.”
• • • • •
To connect with Vivos Therapeutics or any other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.