By Melane Sampson

Closely held Future Fields is spearheading the world’s first synthetic biology system with an innovative transgenic bug-based approach—leveraging fruit flies to combine genetic engineering with insect farming to unlock challenging, customized recombinant proteins at scale.
“Our proprietary EntoEngine platform represents the next evolution in advanced biomanufacturing, meeting the growing demand for recombinant proteins more efficiently, economically, and with a significantly reduced ecological footprint compared to conventional methods,” Matt Anderson-Baron, Ph.D., co-founder and CEO of Future Fields, says in an interview with BioTuesdays.
Recombinant proteins, widely used in research, medicine, and cell culture, are synthesized in laboratories, replicating the amino acid sequences of naturally occurring proteins. These proteins can also be engineered to enhance properties such as solubility and production yield.
“Traditional expression systems such as yeast and E. coli are effective for producing simple proteins but lack the mechanisms needed to produce more complex products,” Dr. Anderson-Baron says. “By contrast, mammalian cells are capable of synthesizing complex proteins but often face significant cost inefficiencies when scaled up.”
Launched in 2018, Future Fields was founded on the belief that biology could solve the world’s biggest problems, from food insecurity to novel treatments for disease, with minimal environmental impact. “We founded the company when the field of cellular agriculture was new and exciting—we just knew we wanted to be involved in some way. Since I have a niche skillset in cell culture, the idea that commoditized food could be created ethically and sustainably with cell culture applications, compared to traditional practices, really resonated with me.”
Early industry challenges, such as high cost and scalability, led Future Fields to adopt a novel approach. “We decided to think outside of the tank and look into how we could manufacture growth factors—recombinant proteins—better, faster, cheaper, and at a much larger scale, which is what led us to investigate an insect approach,” Dr. Anderson-Baron says.
“We spent a lot of time learning to understand the salient pains of the market and how we are best suited to solve them. What we honed in on is unlocking the production of unmakeable proteins,” he adds.
Dr. Anderson-Baron explains that a significant number of proteins cannot be manufactured using traditional protein expression systems. Proteins are functional products that must be correctly synthesized to perform as intended. Traditional systems—yeast, E. coli, and mammalian cells—are all single-cell platforms. This means they rely on one set of biological machinery to produce proteins, which often proves inefficient for generating functionality.
“Forcing hundreds of thousands, if not millions, of different protein types into just three boxes clearly isn’t working,” Dr. Anderson-Baron asserts. “As a result, many promising treatments aren’t making it out of the lab. These therapies cannot be manufactured, even on a small scale, to support drug development and clinical testing. This presents a major challenge—an estimated 50% of all proteins remain unmakeable. Viable solutions are left dying in the dark, their potential unrealized due to technical limitations.”
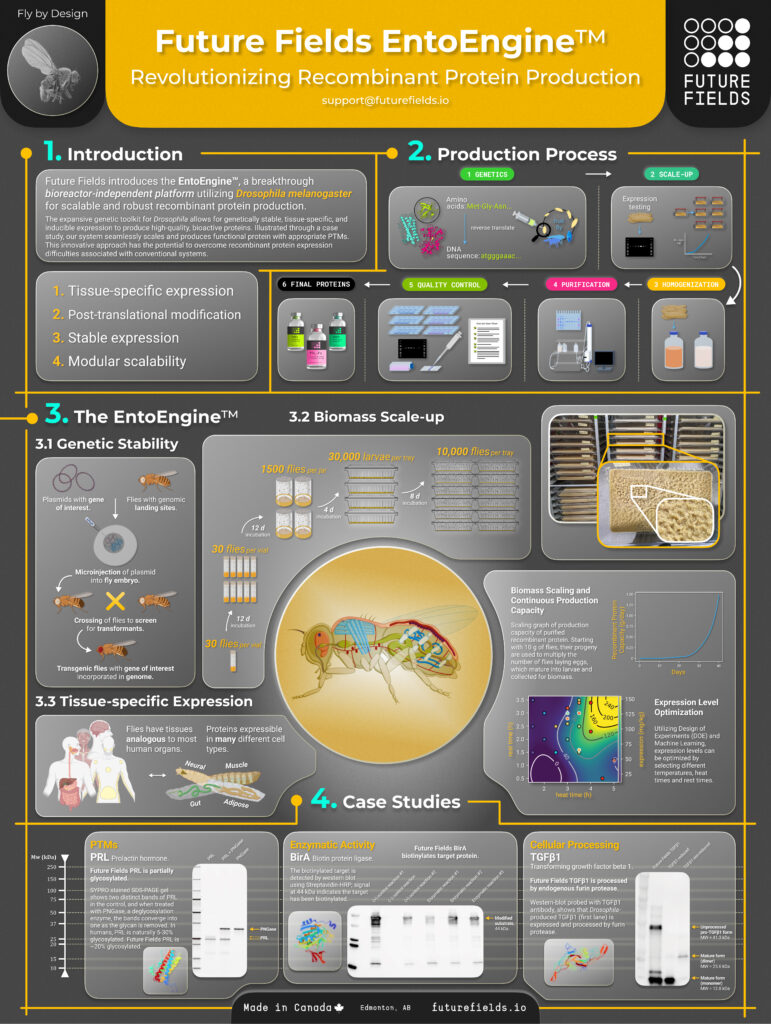
Enter the EntoEngine platform technology, which Dr. Anderson-Baron emphasizes is expanding the biological machinery for protein manufacturing, unlocking the production of previously unmakeable proteins and bringing them closer to clinical use. By enabling the expression of proteins within specific fruit fly cell types, Future Fields is increasing the optionality within the protein production toolkit. This approach targets more than 200 unique cell types, which are essential for producing new and potentially groundbreaking proteins.
“Each one of these cell types has distinct biological machinery that we can harness for protein production,” Dr. Anderson-Baron says. “For example, our EntoEngine is the only system capable of manufacturing proteins in neurons at scale. By targeting protein expression to Drosophila [fruit fly] neurons, we can efficiently manufacture these proteins at scale.”

“What’s unique about our system is that we’re exponentially expanding the protein production toolkit, leveraging it to unlock the production of unmakeable proteins in a way that’s meaningful and enables us to bring critical products to the clinic,” he adds.
Asked why fruit flies, Dr. Anderson-Baron notes their long-standing role in science, “Drosophila, have been used as a model organism for 120 years. We know more about them than we do about arguably any other multi-cellular species. There’s a mountain of data on their genomics, which gives us a unique advantage.”
Future Fields’ EntoEngine platform technology involves inserting a specific protein-coding gene into the genome of fruit fly embryos. This trait is rapidly inherited by offspring, enabling scalable production, Dr. Anderson-Baron points out. Once the strain is established, the insects are reared, harvested, processed, filtered, and clarified to extract lysate for downstream purification. From this point onward, the workflow aligns with the processing of traditional systems.
Leveraging the fruit fly’s rapid reproduction and minimal resource needs, Future Fields’ 6,000-square-foot, state-of-the-art production facility, equipped with clean rooms, is designed to produce significantly more protein in less time while reducing its environmental footprint. “This facility gives us the infrastructure we need to manufacture with high capacity and stringency, enabling us to advance toward GMP manufacturing and access high-value, high-stringency markets, particularly in therapeutics,” Dr. Anderson-Baron contends.
Third-party assessments report the company’s factory emits 86% less carbon and uses 74% less water compared to conventional methods. Additionally, Future Fields donates 1% of its revenue to 1% for the Planet, a global initiative supporting environmental protection.
In 2023, Future Fields’ growth factors became the first in the world to earn the prestigious ACT Environmental Impact Factor Label. The company also achieved Green-certification from My Green Lab, the worldwide gold standard for laboratory sustainability practices. “These recognitions underscore our commitment to delivering high-quality, sustainable recombinant proteins while minimizing environmental impact and contributing to a more sustainable future,” Dr. Anderson-Baron says.
Future Fields’ core business model is contract manufacturing custom proteins for clients ranging from global food and agri-science giants to big pharma. At the same time, the company continues to develop and launch proprietary products to showcase the capabilities of its novel technology.
“We are still a young company, but in the last year, we have established a robust, repeatable process for developing and launching new proteins,” Dr. Anderson-Baron says. “Our unique insect-based approach is solving significant industry challenges and has the potential for massive impact.”
• • • • •
To connect with Future Fields or any other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.