By Melane Sampson

Climb Bio (NASDAQ:CLYM) is advancing a pipeline of best-in-class, patient-tailored therapeutics to address the unmet needs of complex and clinically-diverse immune-mediated diseases.
“Immune-mediated diseases impact one in seven people and are increasing in prevalence worldwide, with many patients suffering from poor outcomes because their disease cannot be controlled with available therapies,” Aoife Brennan, MD, president and CEO of Climb says in an interview with BioTuesdays.
“We believe that we can impact the pathways that cause immune-mediated diseases to give patients precious time free of disease,” she adds.
Dr. Brennan explains that immune-mediated diseases occur when the immune system mistakenly attacks the body’s tissues, which can result in permanent organ damage or even death. “Climb is currently focused on B-cell mediated diseases, a sub-set of immune-mediated diseases. B cells are associated with producing antibodies, which are an essential component of the immune response to infection.”
Patients with B-cell mediated diseases develop antibodies against their own tissues, triggering an immune response that leads to disease progression. More than 2.5 million Americans suffer from a B-cell mediated disease.
Climb’s lead product candidate, budoprutug, is an anti-CD19 monoclonal antibody that has demonstrated B-cell depletion and the potential to treat a broad range of B-cell mediated diseases with doses as low as 100 mg. CD-19 is a cell-surface protein that is expressed on the surface of B cells.
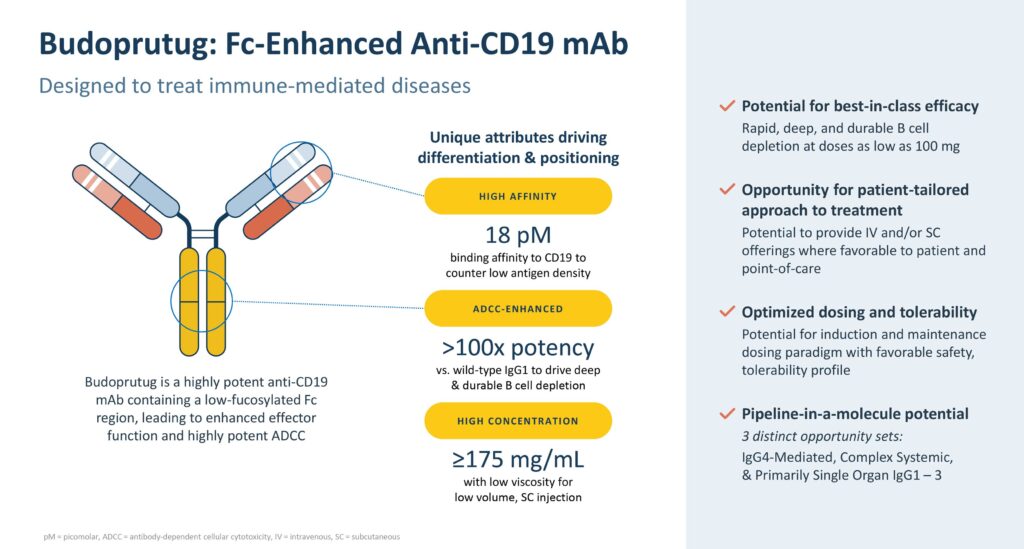
Dr. Brennan notes that budoprutug is designed to deplete CD19-positive B cells, including antibody-secreting cells, to directly reduce pathogenic autoantibodies. It has the potential to modify the course of immune-mediated diseases.
“Budoprutug, or budo for short, binds to CD-19, attracting natural killer cells to destroy or deplete B cells that are impacting disease pathogenesis. So, it gets rid of the bad actors,” she explains.
Modified to improve the potency of the antibody-dependent cellular cytotoxicity (ADCC) component 100-fold compared to a natural IgG1 antibody, budoprutug has been formulated for administration either intravenously or subcutaneously. Depending on the clinical context, this means it could be administered to patients at home, Dr. Brennan highlights.
“We believe CD-19 is an important target because it offers the best therapeutic window of opportunity compared to other potential targets for B-cell mediated diseases. CD-19 is expressed on all B cells except plasma cells, which are responsible for maintaining immunity. So, we want to preserve the plasma cells,” Dr. Brennan asserts. “CD-19 has the best profile for depleting the cells you want to deplete while sparing the cells you want keep for maintaining long lived immunity.”
Budoprutug is currently in development for three different diseases mediated by B cells, including membranous nephropathy (MN), systemic lupus erythematosus (SLE), and immune thrombocytopenia (ITP).
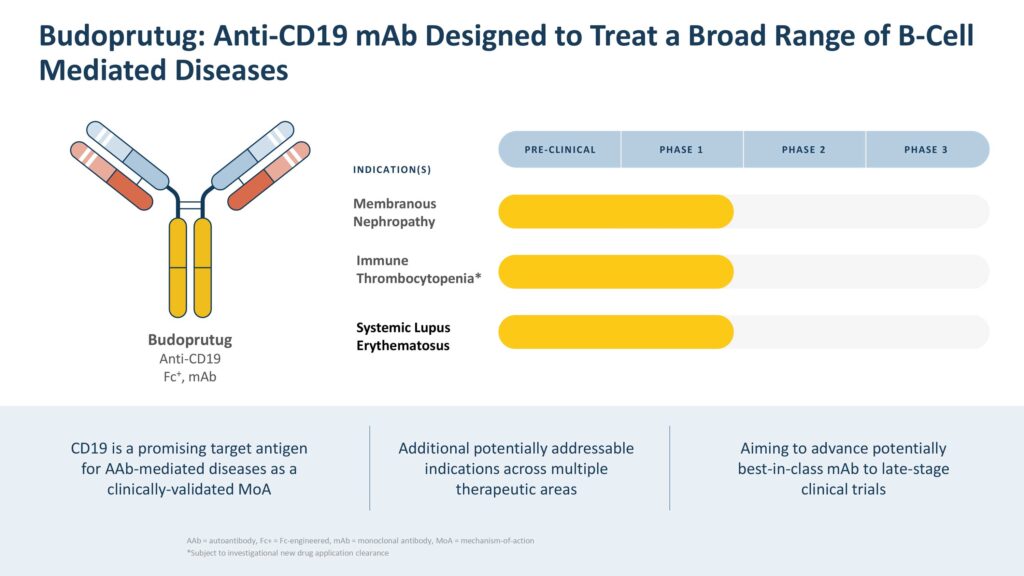
MN, SLE, and ITP are autoimmune disorders that occur when the body’s immune system attacks its own healthy tissues and cells. In MN, the immune system attacks tiny kidney filters called glomeruli, which can cause excess protein in the urine, leading to problems with kidney function. In SLE, various organs and tissues in the body are attacked, often mimicking other diseases, making it hard to diagnose. ITP occurs when the immune system destroys platelets, which are blood cells needed for clotting.
“The reason why we have chosen these three programs is because each of them represents a different category of B-cell mediated diseases,” Dr. Brennan says. “There are many different types of B-cell mediated diseases that can affect almost every organ in the body, but they tend to fall into three categories based on different attributes: IgG4-mediated diseases, single organ rare IgG1-3, and complex systemic diseases. We have selected one indication from each category to help us understand the potential of budo across all diseases in these categories, so there is the potential for a much broader opportunity.”
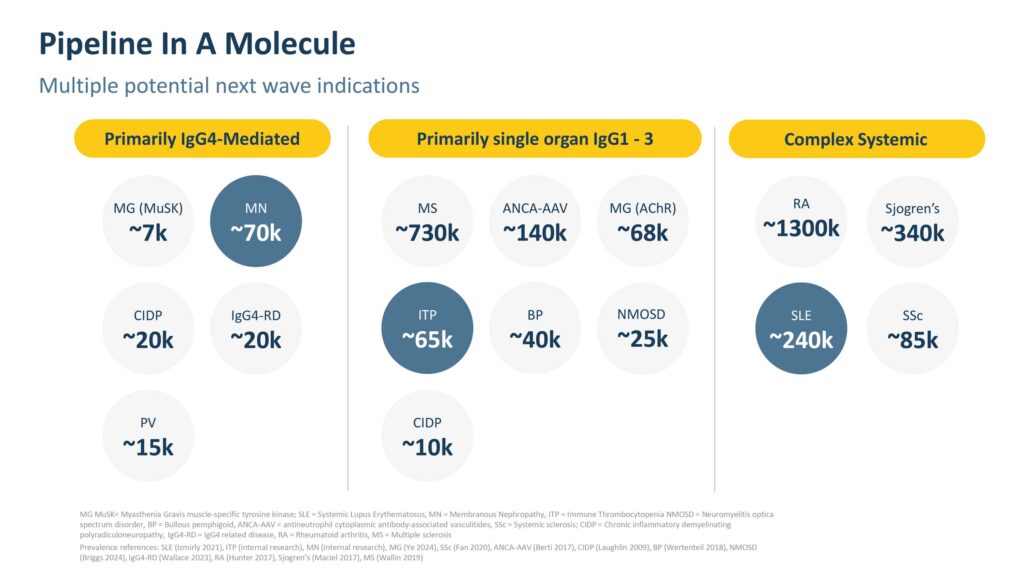
Recently, Climb received FDA clearance of its IND for budoprutug in SLE. “This milestone has enabled us to plan a Phase 1b open-label, intravenous formulation clinical trial of budo in patients with active lupus,” Dr. Brennan says.
Largely effecting young women in the prime of their lives, Dr. Brennan points out that SLE is a very challenging disease to diagnose and manage because it can present in multiple different organs with varying severity, such as the lungs, skin, heart, joints, or even as a miscarriage, without one unifying symptom. This often delays patients from being referred to a rheumatologist for the blood test required to identify lupus as the diagnosis. Approximately 240,000 Americans are currently suffering from SLE, with 10% to 20% of patients being treatment-resistant.
“Lupus is a bit of a graveyard for drug development, with a lot of therapies that have been tried and a lot that have failed,” Dr. Brennan says. “When we looked at all of the treatments that have been tried in lupus, the ones that have tended to work somewhat are the ones that target B cells, giving us the indication that B cells are the driver of this disease.”
“We believe budo can deplete all the B cells, which would go a long way to putting these patients into remission with an injection every six months. This would be transformational for these patients,” she adds.
According to Dr. Brennan, the trial is designed to evaluate the safety and impact of ascending dose regiments on the speed and depth of depletion of circulating B cells, the decline in production of pathogenic autoantibodies, and the nature of B-cell subsets that are produced upon recovery of B cells. In addition, patients will be followed for changes in clinical outcomes. The initiation of the trial is anticipated in the first half of 2025.
Dr. Brennan outlines that Climb was formerly known as Eliem Therapeutics. The company rebranded and transitioned to Climb Bio in October 2024 to reflect its mission to develop a pipeline focused on immune-related diseases, following its acquisition of Tenet Medicines. “The new name pays homage to the journey patients with autoimmune diseases must travel and to the work required to develop new treatments with them in mind.”
“We are a young company, but we are very well-funded through 2027, and we expanded our board and management team with a number of key individuals with diversified experience,” Dr. Brennan says.
“B-cell mediated diseases are really the just start of the journey for us,” she adds. “We have broad ambitions to become a leader, not just in B-cell mediated diseases, but in all immune-mediated diseases by partnering with patients, key opinion leaders, and scientists to identify meaningful insights and better answers that will lead us to new, innovative ways of developing more inspired medicines for patients living with these challenging diseases,” she concludes.
• • • • •
To connect with Climb Bio or any other companies featured on BioTuesdays, send us an email at [email protected].