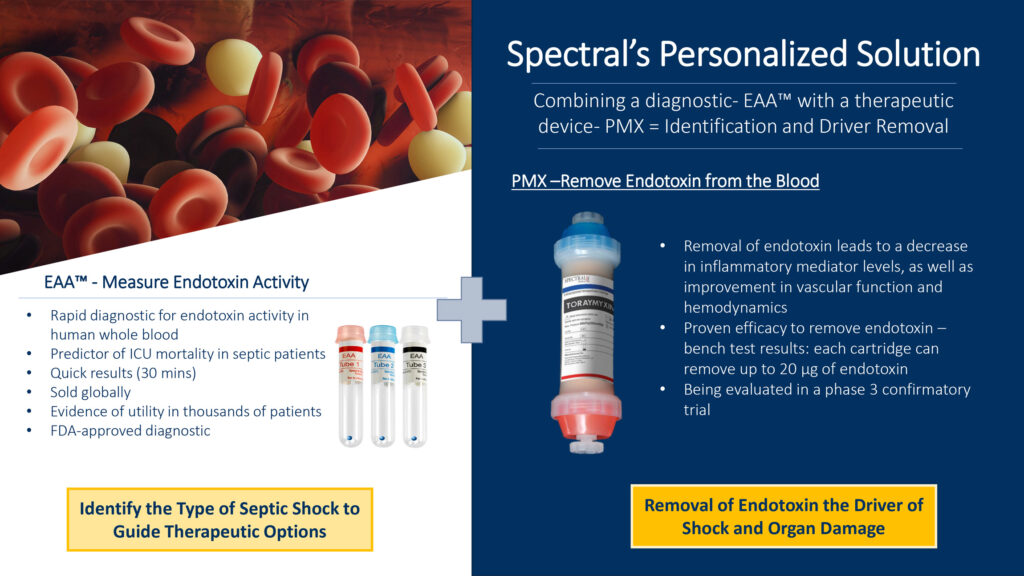
Spectral Medical (TSX:EDT) is seeking to improve outcomes for millions of patients with life-threatening endotoxic septic shock (ESS), by combining its targeted diagnostic, Endotoxin Activity Assay (EAA), with its therapeutic hemoperfusion device, Toraymyxin (PMX), to identify and remove endotoxin from the bloodstream.
“Millions worldwide are affected by septic shock, which can be deadly and is difficult for physicians to diagnose, so there’s a pressing need for a biological marker that can help identify the cause, indicate the severity of the illness, and monitor the impact of treatments,” Chris Seto, chief executive officer of Spectral, says in an interview with BioTuesdays, alongside John Kellum, MD, MCCM, chief medical officer of Spectral.
Mr. Seto explains that sepsis manifests in various forms and stages of severity as a result of the body’s hyper-inflammatory response to acute infection. Spectral is focused on ESS, the most malignant form of sepsis, which affects approximately 140,000 patients each year in the US, and has a mortality rate of more than 50%, and costs the healthcare system billions of dollars in sepsis management annually. ESS is characterized by a dangerous drop in blood pressure and multiple organ failure caused by the presence of a bacterial toxin, known as endotoxin.

Dr. Kellum, a world-renowned expert in sepsis, acute kidney injury, and blood purification, adds that endotoxin, a component of the Gram-negative bacterial cell wall and mainly originating from intestinal bacteria, is one of the main triggers of septic shock and multiple organ failure. There is no approved targeted therapy for ESS patients, who are relegated to standard of care and organ support, such as antibiotics, mechanical ventilation, and dialysis.
“Measuring endotoxin in the bloodstream is very challenging and while it can be done easily in other fluids, those techniques cannot be used for in vivo testing in patients,” Dr. Kellum says. “So, we developed EAA by incorporating the body’s natural mechanisms of endotoxin detection and adapting them into a system that provides an accurate endotoxin measurement within 30 minutes. Until EAA, there was no reliable method to measure endotoxin in the bloodstream.”
Spectral’s EAA, the only Health Canada, FDA cleared, and CE marked diagnostic for endotoxin activity in the world, is regularly utilized in research studies focussing on the role of endotoxin in disease development.
“Combining the diagnostic—EAA—with the intervention—PMX—addresses the root cause of the problem and changes the equation for patients with ESS,” Dr. Kellum suggests. “Guided by EAA, Spectral’s unique therapeutic hemoperfusion device, PMX, is highly effective in removing the sepsis-causing endotoxin from the bloodstream using the electrochemical properties of Polymyxin-B, while avoiding side effects of its systemic administration. The device selectively absorbs circulating endotoxin, rebalancing the immune system leading to a decrease in inflammatory mediator levels and improvement in vascular function and hemodynamics. This device and therapy are poised to become the de facto standard of care for ESS,” he adds.
In 2022, PMX received FDA Breakthrough Device Designation for the treatment of endotoxic septic shock. PMX, available in Canada, where Spectral holds exclusive rights, and is approved for therapeutic use in Japan and Europe, having safely and effectively treated patients more than 340,000 times to date. As of 2019, more than 200 scientific papers and peer-reviewed articles have been published concerning PMX and large databases from Japan show highly significant results.
Clinically available since 1995, Spectral secured exclusive U.S. developmental and commercial rights for PMX in 2009, followed by an exclusive Canadian distribution agreement in 2010. Both EAA and PMX are licensed and available commercially in Canada by Health Canada.
Currently, the company is enrolling patients for its Tigris trial, a Phase 3 confirmatory study designed as a 2:1 randomized trial of 150 patients to evaluate the use of PMX in addition to standard care versus standard care alone in adults treated for endotoxemia and septic shock. “We continue to enjoy very strong activity at our sites and we remain confident in finalizing full Tigris enrollment around year end 2024,” Dr. Kellum says.
Tigris is being conducted at 22 sites throughout the U.S. and is a follow-on trial to the company’s initial EUPHRATES trial, which showed benefit but not statistically significant enough for FDA approval, Mr. Seto says. “However, since the initial trial was robust, and this is a huge unmet need, we analyzed our data further and found that out of a subgroup of 179 patients with certain parameters around organ dysfunction and endotoxic activity level ranges between .6 and .9, there was an approximately 10% absolute mortality benefit. With this evidence, we embarked on the Tigris trial.”
Mr. Seto adds that the Tigris trial design is unique in that the company will build on knowledge gained from the EUPHRATES subgroup of 179 patients and combine it, through Bayesian analysis, with data from the 150-patient confirmatory trial, as part of its FDA regulatory submission.
In terms of commercialization, Mr. Seto informs that in 2020, Spectral entered into an exclusive distribution agreement with Baxter (NYSE: BAX). “There is no better partner for our EAA/PMX device and therapy than Baxter—our device actually runs on their CRRT machine,” he says. “They have a large multi-disciplinary team dedicated to the commercialization launch of our device, and Baxter has a greater than 50% share of the CRRT market in the U.S., making this a natural partnership for us.”
Looking ahead, Mr. Seto emphasizes that Spectral’s 30-year track record of clinical and regulatory excellence lends the company significant confidence in navigating the regulatory pathway for its device and therapy.
“We are highly differentiated and competitive in the ESS space, which currently has no other therapy beyond standard of care, and a U.S. addressable market of more than $2.1 billion annually,” he adds.
• • • • •
To connect with Spectral Medical or any other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.