By Melane Sampson

OSE Immunotherapeutics (ISIN:FR0012127173; Mnemo:OSE) is developing and partnering therapies to control the immune system for immuno-oncology (IO) and immuno-inflammation with first-in-class immunotherapies from target-to-clinic, combined with proprietary research platforms targeting hard-to-treat cancers and diseases with high unmet medical needs.
“We are not a company dedicated to just one technology or asset, but rather, in collaboration with our partners, we are exploring multiple novel therapeutic concepts and novel targets, validating them at the clinical level,” says Nicolas Poirier, Ph.D., CEO and CSO of OSE Immunotherapeutics in an interview with BioTuesdays.
The company’s proprietary drug discovery platforms, Dr. Poirier points out, are central to its strategic objective, which is to deliver next-generation first-in-class immunotherapies. OSE expects to generate significant value from its proprietary platforms.
The BiCKI platform is a bifunctional fusion protein platform focused on IO. OSE’s Myeloid Checkpoint platform is focused on optimizing the therapeutic potential of myeloid cells in IO by targeting immune regulatory receptors expressed by macrophages and dendritic cells. Pro-resolutive mAb is a platform focused on targeting and advancing inflammation resolution and optimizing the therapeutic potential of targeting neutrophils and macrophages in immuno-inflammation. OSE’s mRNA platform enables local delivery into the inflammatory site of innovative immunotherapies encoded by RNA to locally control or suppress immune responses and inflammation.
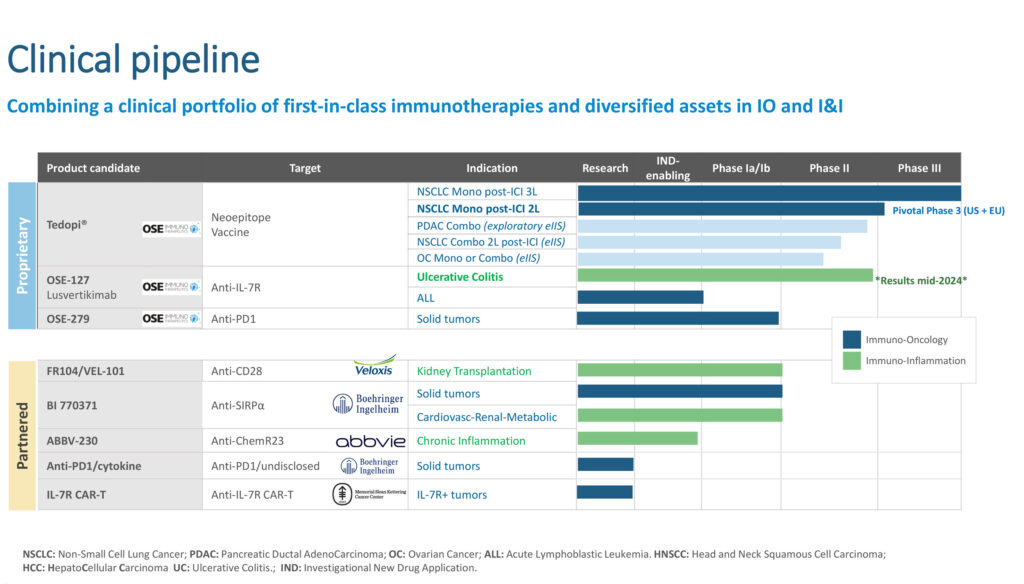
Dr. Poirier explains that OSE’s first-in-class clinical pipeline includes five clinical assets, and the company is working on a sixth. The most advanced product candidate, Tedopi, is an off-the-shelf T-cell epitope-based cancer vaccine monotherapy, targeting five tumor-associated antigens. It is an activating and differentiated immunotherapy expanding tumor specific T-lymphocytes in HLA-A2 positive non-small cell lung cancer (NSCLS) patients.
Lung cancer is the leading cause of cancer mortality worldwide and is the cause of 1.8 million deaths yearly. NSCLC is the most common type of lung cancer, accounting for 85% of all lung cancers.
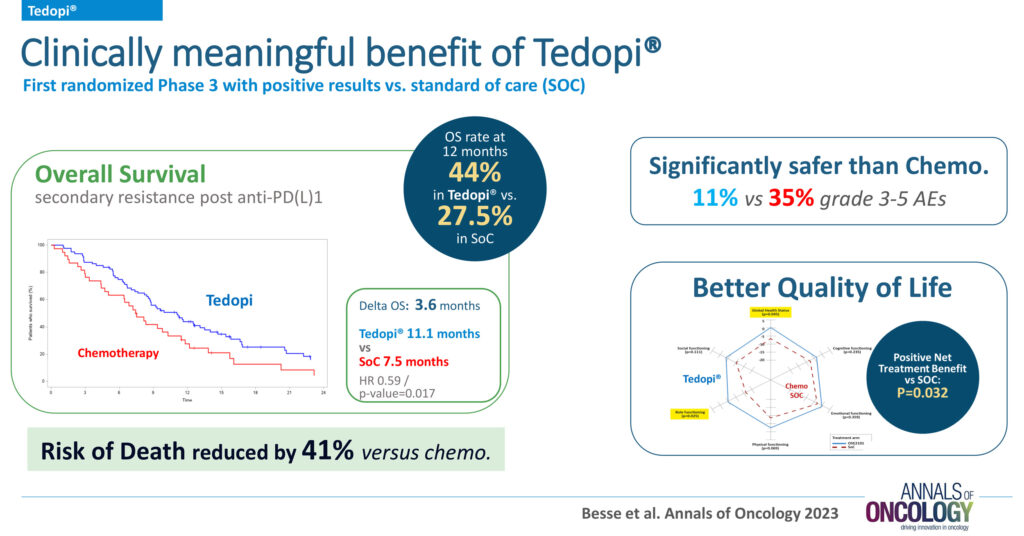
Validated in the top-tier, peer-reviewed journal, Annals of Oncology, Tedopi has demonstrated promising effects with positive Phase 3 results, including final survival, safety, and quality of life, compared to chemotherapy, in NSCLC patients in secondary resistance after checkpoint inhibitor failure. The overall survival rate at 12 months was 44% with Tedopi versus 27.5% with the standard of care. The risk of death was reduced by 41% with Tedopi versus chemotherapy.
“Tedopi is the most advanced therapeutic cancer vaccine in clinical development today, and we believe OSE may very well be the first to commercialize this technology in 2027/28,” Dr. Poirier contends. “We are very excited about the potential of Tedopi as the new standard of treatment for patients with NSCLC who are failing after immunotherapy and currently don’t have any approved therapeutic options.”
Dr. Poirier notes that Phase 2 trials in combination with immunotherapy or chemotherapy, sponsored by clinical oncology groups, of Tedopi in pancreatic and ovarian cancer solid tumors are ongoing.
Recently, OSE announced positive efficacy results for another product candidate, Lusvertikimab, in its proof-of-concept Phase 2 trial for the treatment of moderate-to-severe ulcerative colitis. Lusvertikimab, a pure interleukin 7 antaganist, is a potential first-in-class therapy with novel therapeutic actions based in its differentiated mechanism of action. Dr. Poirier notes that study results showed a favorable safety and tolerability profile in the whole patient population across the two doses tested and during the open label phase of the treatment.
Highlighting that 3.3 million patients across the U.S., Europe, and Japan alone are ulcerative colitis sufferers, Dr. Poirier says it is a disabling, chronic relapsing inflammatory bowel disease (IBD) with a patient population in regular need of new therapies. “We are very excited about this new technology, and we are one of very few companies leading the IBD space, providing a new class of drugs and new therapeutic options for patients with positive signal.”
“We are optimistic about the potential of Lusvertikimab for these patients, and the positive clinical efficacy and safety results from this Phase 2 study represent a strong catalyst for future opportunities, enhancing OSE’s presence in this growing field of chronic immune inflammation,” he adds.
“Not only is there a significant opportunity for OSE’s Lusvertikimab in ulcerative colitis, but also in the acute lymphoblastic leukemia target market,” Dr. Poirier says. “Acute lymphoblastic leukemia is a rare disease with diagnosed incident cases in Europe, China, and Japan estimated to reach 26,482 by 2029.”
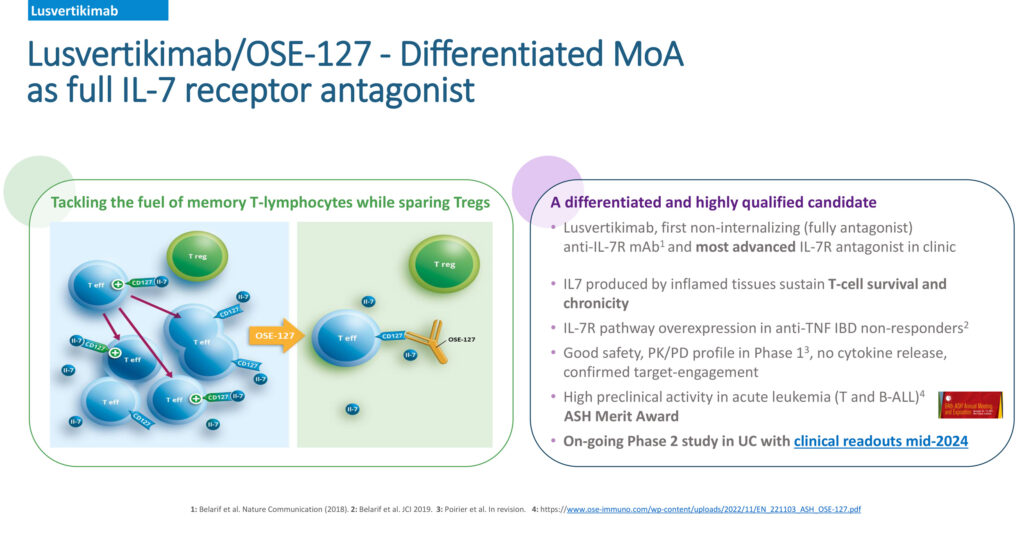
In a collaborative research program between OSE and the University Medical Center Schleswig-Holstein, Dr. Poirier informs that preclinical data on the use of Lusvertikimab, a potential novel immunotherapy option for the treatment of B- and T-Cell lymphoblastic leukemia, was recently published in the top-tier, peer-reviewed hematology field journal, Blood. “Novel targeted immunotherapy treatments are urgently needed for these patients, and we are happy to face this clinical challenge in partnership with research leaders at the University of Kiel.”
Dr. Poirier outlines that OSE’s product development is supported by an international network of significant partnerships with pharmaceutical leaders, clinical organizations, and academic laboratories, which is fundamental to the company’s long-term strategy and daily operations. OSE’s strategic partnerships with global industry leaders, such as AbbVie, Boehringer Ingelheim, Veloxis Pharmaceuticals, and Chong Kun Dang, have yielded more than €200 million thus far, with potential revenue of up to €2.1 billion in milestones.
“Our selective partnerships are critical to our business model, significantly contributing to the cash flow required to continue discovering and developing new targets and therapeutic candidates for unmet medical needs,” he adds.
Looking ahead, Dr. Poirier says, “OSE’s plan is to continue to build a leading immunotherapy company by sharing knowledge with existing and new partners and attracting the expertise required to develop breakthrough innovations and drive the rapid delivery of new therapies to patients in need.”
• • • • •
To connect with OSE Immunotherapeutics or any other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.