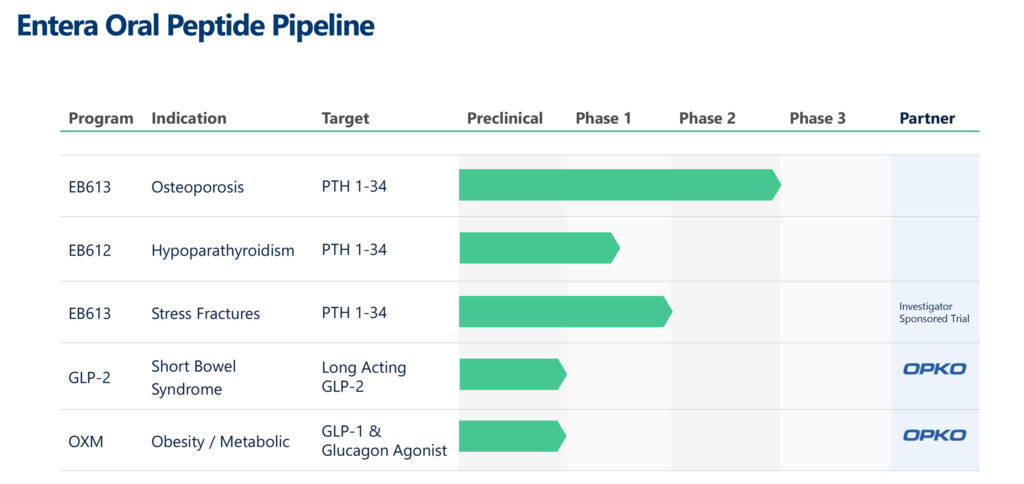
Entera Bio (NASDAQ:ENTX) is leveraging a disruptive technology platform called N-Tab to develop first-in-class, once-daily oral tablets of peptides and small therapeutic protein replacement therapies. The company currently has five programs at various stages of development, aiming to simplify care for millions of patients living with osteoporosis, hypoparathyroidism, obesity, and short bowel syndrome.
“N-Tab is not a ubiquitous platform, therefore, each of our oral peptide programs has been selected for technological alignment and its potential to deliver a valuable peptide treatment orally so patients can adequately manage their conditions and live healthier, injection-free lives,” Miranda Toledano, CEO of Entera Bio, says in an interview with BioTuesdays.
“Our two lead indications focus on underserved conditions that disproportionately afflict women in perimenopause and post-menopause,” she explains.
Entera’s lead clinical candidate, EB613, is being developed as the first osteoanabolic (bone building), mini-tablet treatment of PTH (1-34) dedicated to post-menopausal women with osteoporosis. A pivotal, Phase 3 study of EB613 is planned to start in the first half of 2025.
Recently, positive data from the placebo-controlled Phase 2 study of EB613 in 161 post-menopausal women with low bone mineral density (BMD) or osteoporosis was published in the top-tier, peer-reviewed Journal of Bone and Mineral Research. Results demonstrated that EB613 was well tolerated and produced a dually acting pharmacodynamic profile, increasing key markers of bone formation (P1NP) while simultaneously mildly suppressing markers of bone breakdown, also known as resorption (CTX).
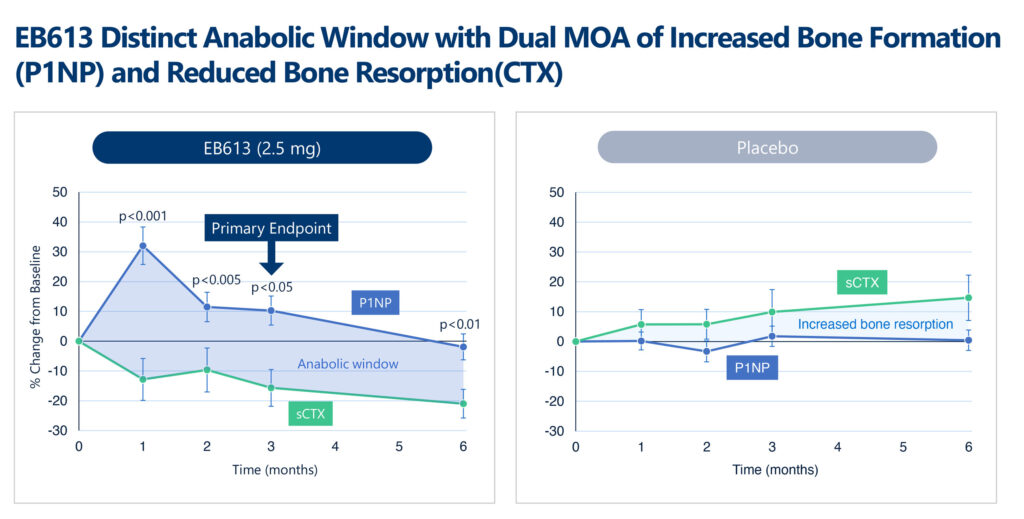
“Mechanistically, EB613 appears to stimulate new bone formation on trabecular and, more so, on cortical bone or hip and femur, by preferential stimulation of osteoblastic activity while also reducing osteoclastic activity, which is very compelling,” Ms. Toledano contends.
“Significant gains in BMD, especially at the hip region, were observed at the end of the six-month study, with no significant safety concerns. Notably, this is a different pattern of response or differentiated anabolic window compared to Forteo, a daily SC PTH(1-34) teriparatide injection, which we will continue to evaluate in our phase 3 study,” she adds.
EB613 stands as the first program to potentially benefit from a key change at the U.S. Food and Drug Administration (FDA) concerning the regulatory endpoint for osteoporosis drugs.
By January 2025, the FDA is expected to rule on the American Society for Bone and Mineral Research and the Foundation for the National Institutes of Health Study to Advance Bone Mineral Density as a Regulatory Endpoint (ASBMR-FNIH SABRE) application to approve BMD as a surrogate endpoint for fractures, to be used in future trials of new osteoporosis drugs.
Ms. Toledano outlines that BMD is the first surrogate endpoint undergoing qualification by the FDA under the 21st Century Cures Act, which was signed into law on December 13, 2016, to help accelerate medical product development and bring new innovations and advances to patients who need them faster and more efficiently. Historically, FDA approval of new anti-osteoporosis drugs required fracture (bones breaking) outcomes as the regulatory endpoint, which demanded relatively large and long studies with ethical concerns. No new drug has been approved in osteoporosis since 2019.
“We view the FDA’s BMD endpoint ruling as a major catalyst for EB613, and this will be a big win for innovation in the field of osteoporosis in general. We are especially keen to start our pivotal study of EB613 in a much wider, at-risk population of women with osteoporosis where injectable anabolic drugs do not play a dominant role,” Ms. Toledano says.
Osteoporosis is a progressive bone disease marked by low bone mass and skeletal architecture deterioration, resulting in increased bone fragility and fracture risk. More than 200 million women worldwide are affected by osteoporosis, surpassing the combined total of those affected by heart attack, stroke, and breast cancer.
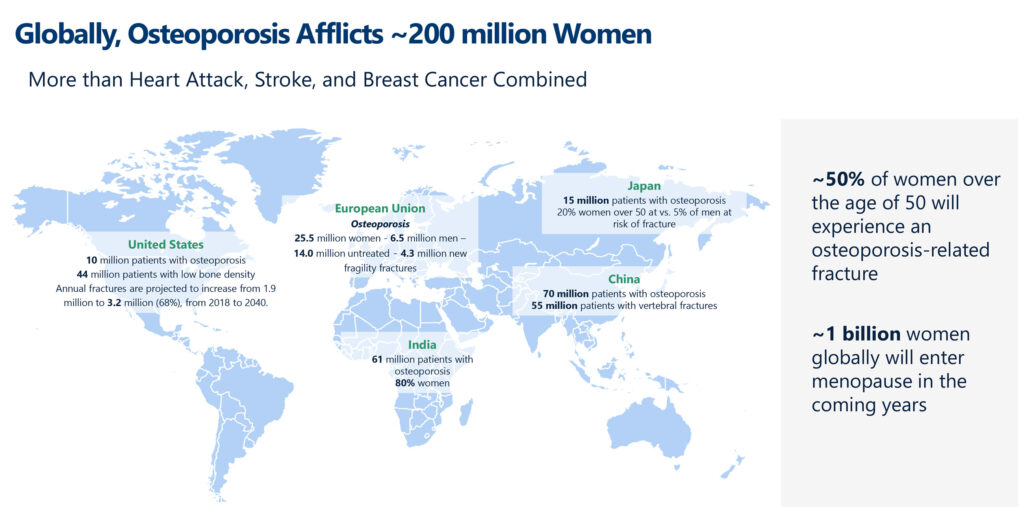
“Fracture rates are not declining, and menopause is occurring much earlier in life,” Ms. Toledano highlights. “Fifty percent of women over the age of 50 are at risk of fragility fractures and approximately 20% of adults will die within the year following a hip fracture. Osteoporosis continues to be a high-risk, silent disease with real unmet needs.”
Current osteoporosis pharmacologic treatments involve one of two main mechanisms of action. The vast majority of patients are treated with agents that supress bone degradation (anti-resorptive agents), either orally or with a once-a-year IV injectable bisphosphonate or with Prolia (denosumab), which requires only two injections a year, she adds.
Nonetheless, Ms. Toledano points out that AACE, Endocrinology Society, NAMS, and other treatment guidelines recommend that women with osteoporosis at higher risk for fracture receive anabolic therapy as initial therapy or as second-line treatment in women who have a suboptimal response to other medications.
“Scientifically, there is ample data to support the use of anabolics earlier in the treatment paradigm followed by anti-resorptive therapy. However, approved injectable anabolic treatments currently require daily subcutaneous administrations such as Forteo or Tymlos, or monthly in-clinic injections such as Evenity, limiting patient acceptance. There are no oral anabolic treatments for osteoporosis on the market today,” she adds.
“We are developing EB613 to support earlier osteoanabolic intervention for high-risk post-menopausal women with osteoporosis. Because of its potential dual mechanism of action, faster onset of action as an anabolic boosting agent, and oral mini-tablet format, we believe EB613 is uniquely positioned to address the needs of millions of women with osteoporosis globally,” Ms. Toledano says.
• • • • •
To connect with Entera Bio or any other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.