By Melane Sampson

BerGenBio (OSE:BGBIO) is developing a pipeline of transformative drugs targeting AXL as a cornerstone of therapy for aggressive diseases, including cancer and severe respiratory infections.
“BerGenBio is a clinical-stage biopharmaceutical company with a solid leadership position in understanding the role of AXL as a promising target to meet life-threatening diseases. We are currently advancing a highly selective and potent AXL inhibitor called bemcentinib,” Martin Olin, CEO of BerGenBio, says in an interview with BioTuesdays.
BerGenBio’s lead compound, bemcentinib, is a first-in-class, selective, oral once-a-day inhibitor of AXL receptor tyrosine kinase (AXL), a potential therapeutic target for serious diseases. Recent studies have demonstrated a central role of AXL signaling in cancer tumor proliferation, survival, metastasis, and resistance to therapy.
AXL is expressed and activated in response to oxidative stress, inflammation, hypoxia, and drug treatment, resulting in a number of harmful effects in cancer and severe respiratory infections, Mr. Olin says. AXL expression has been established as a negative prognostic factor in a large variety of cancers, with compelling evidence in non-small cell lung cancer (NSCLC).
Mr. Olin explains that AXL is a cell surface protein that sits on cells throughout the body and is known to be critical in the evolvement of severe diseases, such as respiratory infections and various cancer indications. AXL plays a key role in the ability of viruses to enter cells and replicate, weakens helpful immune responses attempting to fight off the virus, and slows the repair of damaged tissue.
Mr. Olin says the company is entirely focused on advancing bemcentinib in NSCLC, specifically in front-line therapy for a patient population that has a mutation in a gene called STK11.
“The most meaningful target indication for bemcentinib is NSCLC in patients with the STK11 mutation because their immune systems are highly compromised and AXL is over-expressed in 80% of this patient population compared to 40% to 50% of NSCLC patients without the STK11 gene mutation,” he adds.
Approximately 20% of all NSCLC patients have the STK11 mutation, which is associated with poor overall survival due to rapid tumor cell growth and little or no response to standard of care.
NSCLC is the leading cause of cancer deaths worldwide with a five-year survival rate of less than 25%, largely due to the fact that the majority of patients are being diagnosed after the disease has metastasized and surgical removal is not an option, Mr. Olin outlines.
“The high unmet medical need in the STK11m NSCLC patient population is widely acknowledged, and bemcentinib has received fast track designation by the FDA,” he adds.
BerGenBio’s preliminary clinical and preclinical data indicate that bemcentinib, when combined with standard of care therapies including immune checkpoint inhibitors (ICI) and doublet chemotherapy, may improve the ability of this large, underserved population to activate tumor killing T-cells, potentially improving response to treatment, Mr. Olin contends.
“Bemcentinib is able to play a dual role in inhibiting AXL expression on the tumor cells as well as on immune cells. When added to standard of care, bemcentinib delays resistance to chemotherapy, improves response to ICI, restores anti-PD-L1 response, and extends overall survival,” he adds.
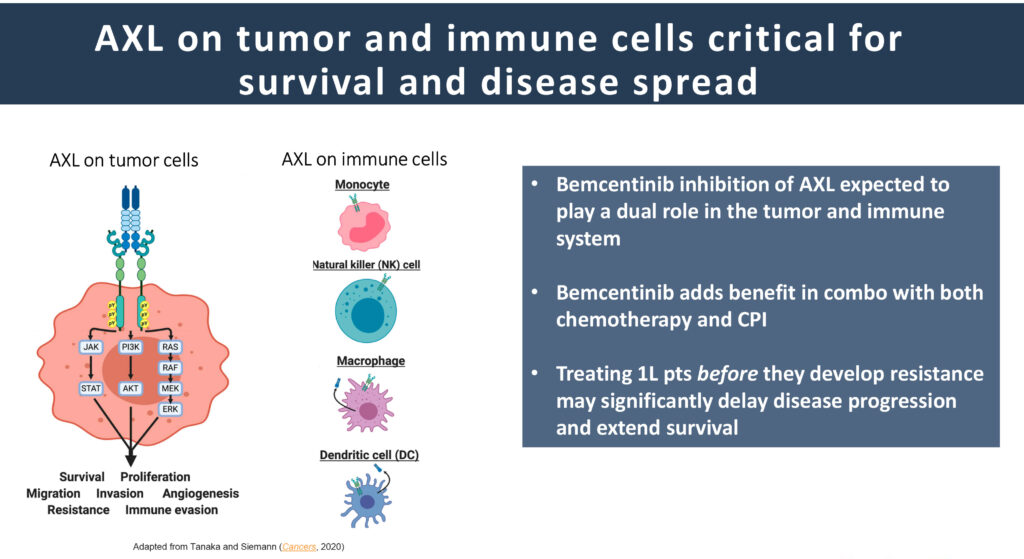
Last year, the company reported positive outcomes from two Phase 2 studies in second-line NSCLC patients with elevated AXL expression regardless of PD-L1 status. Mr. Olin says progression-free survival and overall survival rates were well above what is expected in this patient population when bemcentinib was added to either chemotherapy or Keytruda, an ICI.
“The preclinical and clinical data continues to support the hypothesis that first-line therapy with bemcentinib is highly relevant. Because of the critical role that AXL plays, it is beneficial to inhibit AXL expression before the patient has developed resistance. Early intervention with bemcentinib in first-line therapy, prior to development of resistance, can significantly improve clinical outcome for these patients,” he adds.
With a positive recommendation from the independent Drug Safety Monitoring Board (DSMB), which evaluated the safety of bemcentinib combined with standard of care therapies, including Keytruda and doublet chemotherapy, in first-line STK11m NSCLC patients during a Phase 1b clinical trial, BerGenBio is initiating the Phase 2a portion of the study to establish dosing regimens and evaluate the clinical efficacy of bemcentinib.
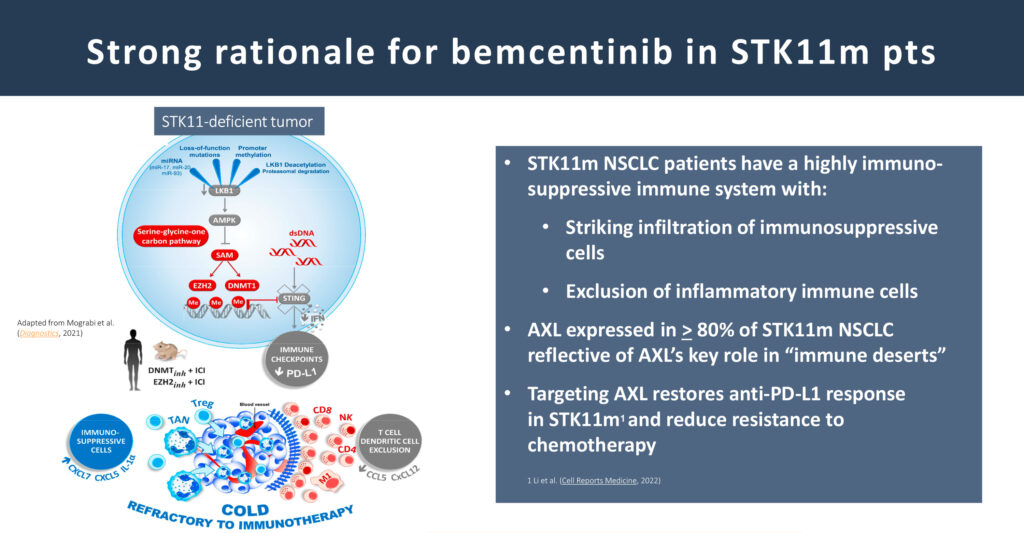
“With these results and given the unmet medical need, it’s not surprising that we are seeing very high interest from the medical community driven by the fact that we really don’t have any treatment options on the market specifically for these patients,” he adds.
Moreover, Mr. Olin underlines that BerGenBio has not observed an escape mechanism with bemcentinib and has noted a number of unique benefits, including a concentration in the lung tissue by 40-fold over other tissues, a critical benefit when treating NSCLC. “Bemcentinib has also proven to cross the blood-brain barrier, which is very important as about 20% of STK11m NSCLC patients have brain metastases when they are first diagnosed.”
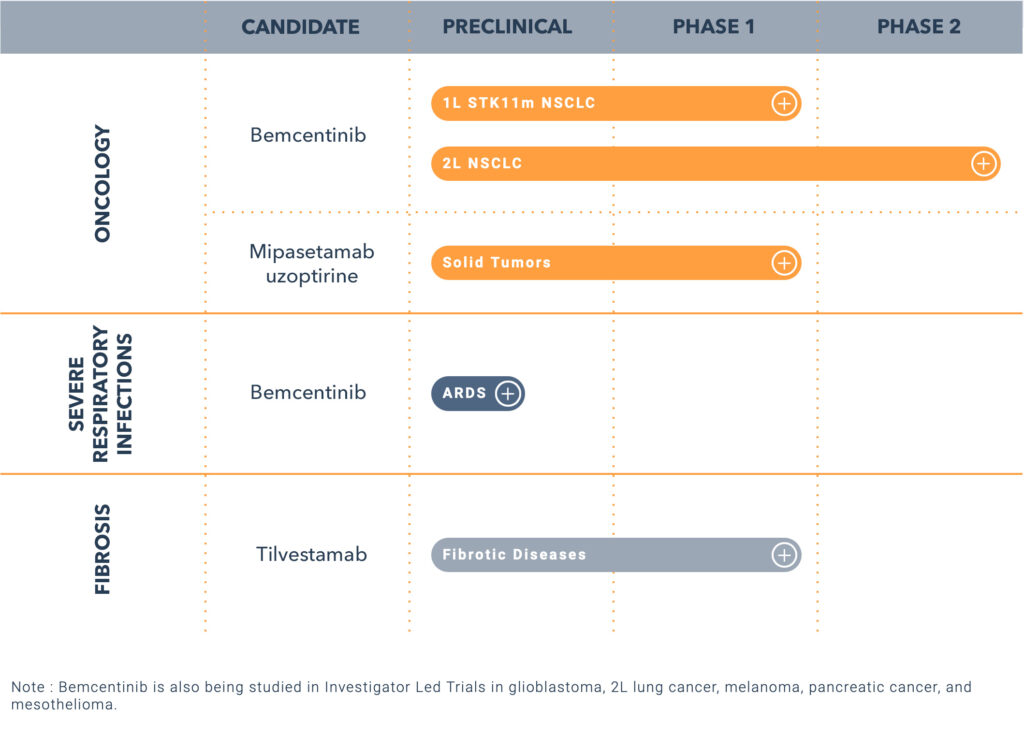
Mr. Olin informs that BerGenBio has studied bemcentinib in more than 600 patients in a variety of diseases, including COVID-19, and has observed the demonstration of monotherapy activity in multiple indications including acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS) liquid tumors.
Recently, the company announced that bemcentinib was selected for inclusion in a National Cancer Institute (NCI) funded study in clinical collaboration with the Mays Cancer Center at UT Health, San Antonio, and Swedish Orphan Biovitrum AV (Sobi). The clinical trial agreement focuses on the study of bemcentinib in combination with Sobi’s pacritinib in patients with advanced lung adenocarcinoma.
Mr. Olin highlights that BerGenBio is collaborating with one of the leading providers of next-generation sequencing technologies, which will provide a synthetic control arm utilizing real-world data to accurately screen patients for clinical trial eligibility as well as access to large U.S. medical centers. “Clinical trials are extremely costly and lengthy so we are very excited about streamlining this process with our new collaborator.”
Looking forward, Mr. Olin says BerGenBio will continue to focus on optimizing its research and helping patients with terminal diseases achieve better outcomes.”
• • • • •
To connect with BerGenBio or any other companies featured on BioTuesdays, send us an email at editor@biotuesdays.com.