By Melane Sampson

Candel Therapeutics (NASDAQ:CADL) is developing off-the-shelf multimodal viral immunotherapies for individualized systemic anti-tumor immune response, specific to the patient’s difficult-to-treat cancer, improving survival while maintaining the quality of life.
“Candel is harnessing the power of viral immunotherapies to kill tumor cells, modify the tumor microenvironment, and generate systemic immune activation against tumor-specific antigens, ensuring cancer patients have efficacious and well-tolerated treatments across disease stages,” Paul Peter Tak, M.D., Ph.D., FMedSci, president and CEO of Candel Therapeutics, says in an interview with BioTuesdays.
Dr. Tak explains that the cancer treatment paradigm has shifted dramatically in light of evidence that the immune system can be educated to recognize and eliminate a patient’s tumor cells. “Our focus is on developing viral immunotherapies for difficult to treat cancers with significant unmet need, for example, patients with early, localized prostate cancer where no new treatment has been approved in more than 30 years, or patients with non-small cell lung cancer (NSCLC) who have failed standard of care chemotherapy and immune checkpoint inhibitor (ICI) treatment.”
Candel has established two clinical-stage multimodal biological immunotherapy platforms utilizing novel, genetically modified gene constructs – one from adenovirus and the other from herpes simplex virus (HSV).
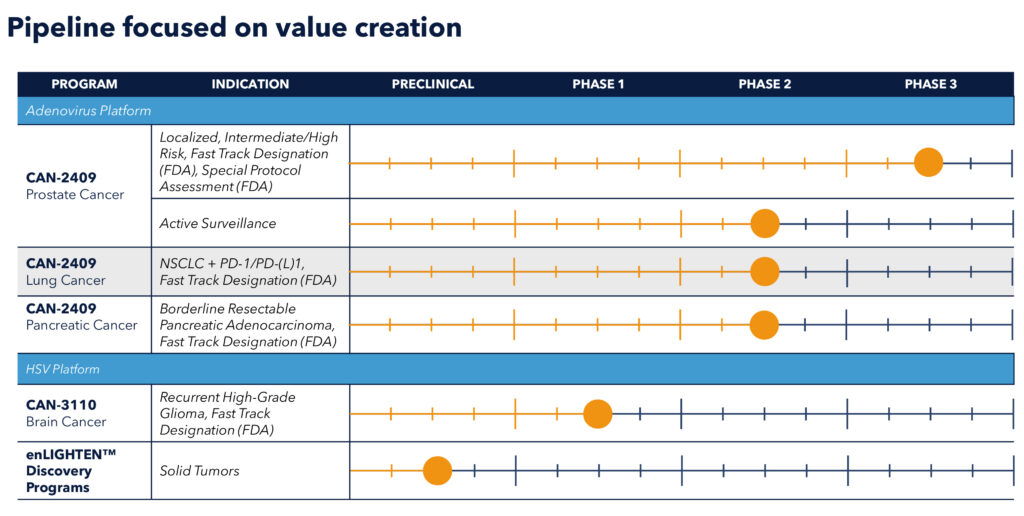
The company’s most advanced candidate, CAN-2409 from the adenovirus platform, is currently in multiple ongoing clinical trials, including an open-label Phase 2a study in NSCLC, a randomized Phase 2a study in borderline resectable pancreatic ductal adenocarcinoma (PDAC), as well as randomized Phase 2b and Phase 3 studies in localized, non-metastatic prostate cancer.
Candel has engineered CAN-2409 to deliver a gene-encoding the HSV-thymidine kinase enzyme into tumor cells. The enzyme then converts valacyclovir, an FDA-approved oral small molecule drug, from its inactive pro-drug state into a form that is toxic to cancer cells. “The incorporation of activated valacyclovir into tumor cell DNA results in the termination of DNA synthesis and permanent cancer cell damage and death,” Dr. Tak says.
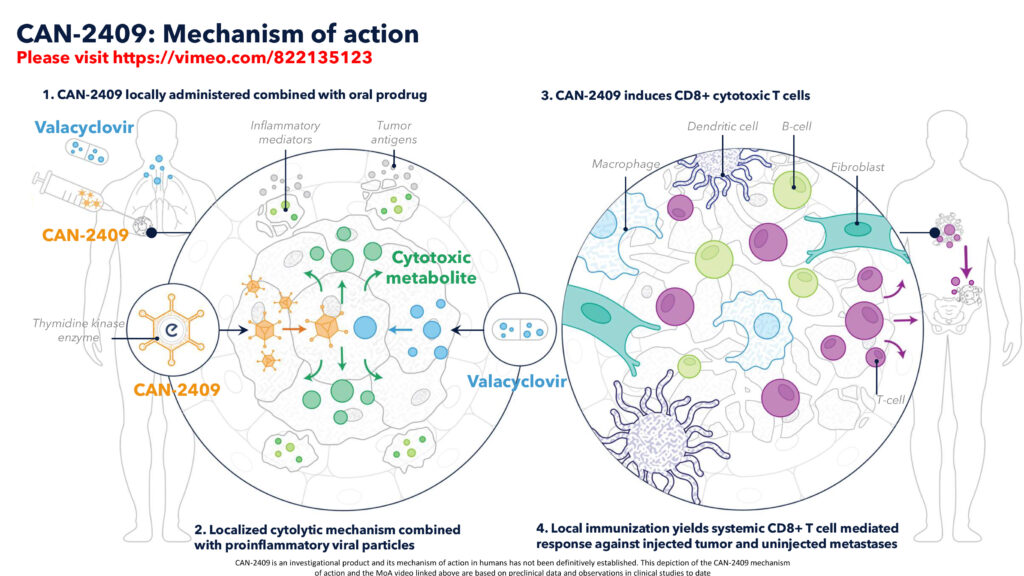
When administered together, CAN-2409 and valacyclovir cause immunogenic cell death, where tumor cells release tumor-specific antigens that are recognized by the immune system. Simultaneously, the adenovirus provokes the release of inflammatory mediators that recruit immune cells which are educated on how to recognize the patient’s tumor neo-antigens.
“CAN-2409 is a systemic immunotherapy delivered locally, which means you don’t need to inject all of the tumors in the patient with metastatic disease,” Dr. Tak contends. “We just choose one or two to educate the patient’s own T cells on how to recognize the tumors throughout the body, with the entire procedure completed via bronchoscopic injection in an outpatient clinic setting within 30 minutes.”
Topline overall survival data from the Phase 2 clinical trial of two administrations of CAN-2409 plus valacyclovir together with standard of care ICI therapy in patients with stage III/IV NSCLC non-responsive to ICI, anti-PD-(L)1, therapy, was presented at the 2024 American Society of Clinical Oncology (ASCO) annual meeting.
“We are very encouraged by the median overall survival (mOS) of 20.6 months after two administrations of CAN-2409 plus valacyclovir in patients with NSCLC whose disease had progressed despite receiving prior anti-PD(L)1 treatment,” Dr. Tak says.
In contrast, a 2022 publication of a clinical trial in a similar population reported that mOS in the control arm that received standard-of-care docetaxel-based chemotherapy was 11.6 months, he adds.
“We have administered CAN-2409 to more than 1000 patients in a range of solid tumor indications in ongoing clinical trials, so we have enormous safety data and we know it is generally well-tolerated,” Dr. Tak says.
CAN-2409 has received fast-track designation from the FDA for every indication the company is pursuing as well as orphan drug designation, most recently, in borderline resectable PDAC.
In the Phase 2 clinical trial in patients with borderline resectable PDAC receiving optimum standard of care plus two to three administrations of CAN-2409, researchers observed mOS of 28.8 months in the active treatment group with a 70% survival rate at two years compared with mOS of 12.5 months in the control group with only 17% survival rate at two years.
“We have data showing that we fundamentally changed the microarchitecture of the tumor,” Dr. Tak says. “This is very encouraging data and consistent with our lung cancer data suggesting that CAN-2409 is well-tolerated and improves survival.”
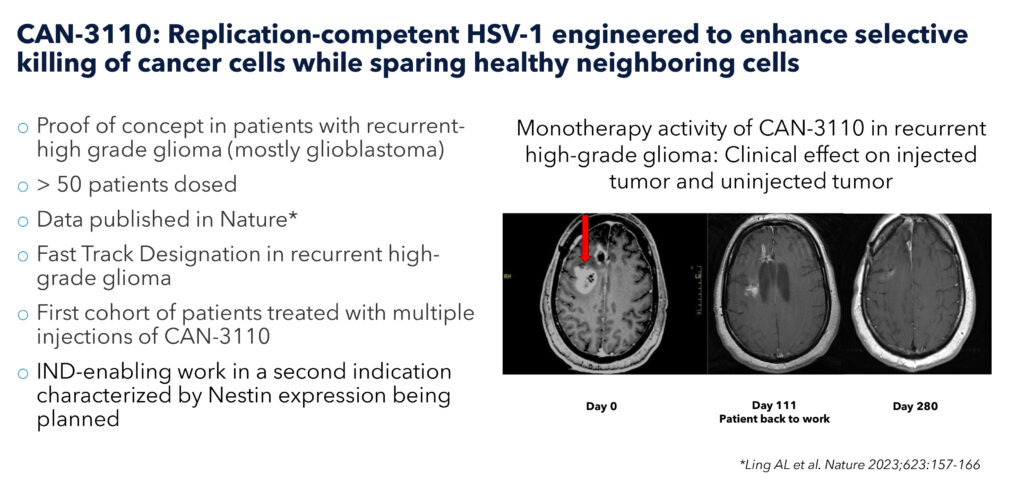
Candel’s lead product candidate from the HSV platform, CAN-3110, is a next-generation, first-in-class, replication-competent HSV-1 oncolytic viral immunotherapy designed with dual activity for oncolysis and immune activation in a single therapeutic.
CAN-3110 is currently in an ongoing Phase 1b clinical trial in patients with recurrent high-grade glioma (rHGG). “These patients have failed neurosurgical resection, chemotherapy, and radiotherapy so they have run out of options, and many of them have months or even just weeks to live,” Dr. Tak contends.
Highlighting that CAN-3110 kills cancer cells while sparing healthy neighbouring cells, Dr. Tak says it is engineered for selective replication placing the gene controlling HSV replication under the control of the Nestin promoter, which is highly expressed in rHGG cells. He points out that Nestin expression has also been detected in aggressive tumors other than rHGG, for example, melanoma, triple-negative breast cancer, and gastrointestinal tumors, which broadens the possibility of expanding the use of CAN-3110 into other indications.
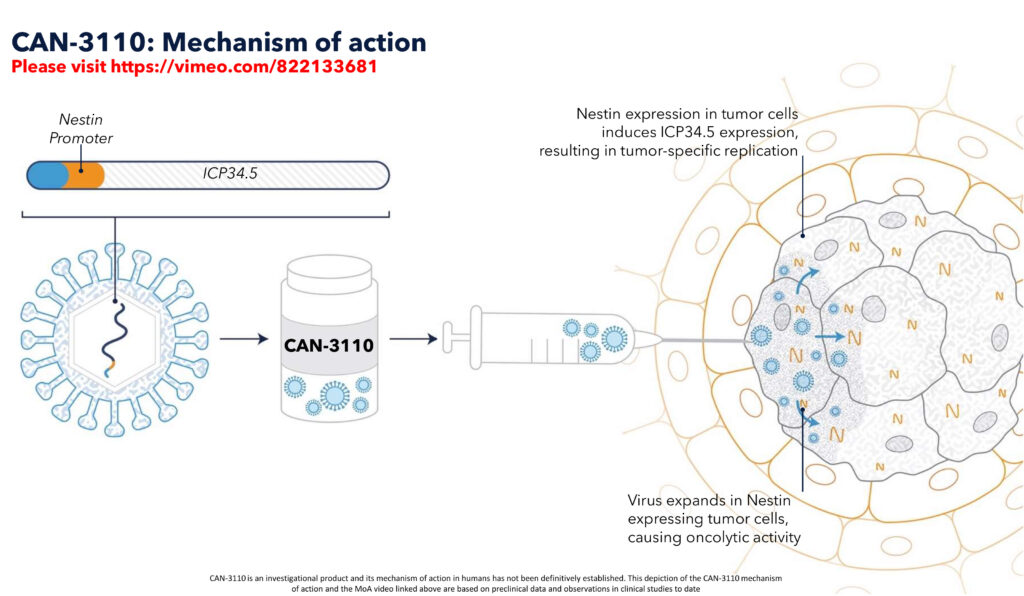
Last October, data, based on the first 41 patients in the CAN-3110 Phase 1b study in rHGG, published in the top-tier, peer-reviewed journal, Nature, demonstrated that there was no dose-limiting toxicity with this approach and expected mOS was doubled with a single injection.
“Nestin is absent in healthy adult brain cells, which may explain why dose-limiting toxicity was not observed with a single injection of CAN-3110 into the brain tumor in patients with recurrent high-grade glioma,” Dr. Tak says.
Recently, CAN-3110 received fast-tracked designation and orphan drug designation from the FDA.
“Since our first results were published, we have introduced CAN-3110 into nine more patients with exactly the same findings,” Dr. Tak reports. “Seeing the same results in 50 patients with just one injection is very meaningful and we have next started to ask the question, what would results look like with multiple injections?”
During ASCO 2024, a poster presentation focused on an additional six patients participating in an ongoing CAN-3110 clinical trial in rHGG demonstrated the feasibility of up to six injections that were found to be generally well-tolerated by patients, Dr. Tak informs. “We expect to present the first data for these six patients in the second half of 2024.”
Dr. Tak recalls a particular patient with two brain tumors who had failed neurosurgical resection, chemotherapy, radiotherapy and was refusing all standard-of-care options but did agree to a single injection of CAN-3110. “This patient had accepted that he was at the end of life, however, by day 111, after receiving CAN-3110, he was back to work.”
Also HSV-based, is Candel’s preclinical enLIGHTEN Discovery Platform, which Dr. Tak describes as a systemic, iterative discovery platform leveraging human biology and advanced analytics to create new viral immunotherapies for solid tumors. In a discovery partnership with the University of Pennsylvania, the company aims to overcome barriers to CAR-T therapies leveraging newly engineered viruses potentially resulting in improved survival rates.
Dr. Tak says the company looks forward to topline readouts of Phase 2 and Phase 3 CAN-2409 prostate cancer trials later in 2024.
“The science is really strong, and while it’s a new modality, we understand how it works and we have delivered the data to support this groundbreaking vaccination,” Dr. Tak says. “We are getting ready to start large, randomized trials of CAN-2409 in NSCLC and PDAC. Providing effective viral immunotherapies to as many patients who need them as possible – that’s our dream.”
• • • • •
To connect with Candel Therapeutics or any other companies featured on BioTuesdays, send us an email at [email protected].